Tarso B. Ledur Kist - Open and Toroidal Electrophoresis
Здесь есть возможность читать онлайн «Tarso B. Ledur Kist - Open and Toroidal Electrophoresis» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Open and Toroidal Electrophoresis
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
Open and Toroidal Electrophoresis: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Open and Toroidal Electrophoresis»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
The exact expressions of separation efficiency, resolution, peak capacity, and many other performance indicators of the open and toroidal layouts are presented and compared.
Featuring numerous illustrations throughout,
offers chapters covering: Solvents and Buffer Solutions; Fundamentals of Electrophoresis; Open Layout; and Toroidal Layout. Confronting Performance Indicators is next, followed by chapters on High Voltage Modules and Distributors; Heat Removal and Temperature Control; and Detectors. The book finishes with an examination of the applications of Toroidal Electrophoresis.
The first book to offer a detailed account of Toroidal Electrophoresis—written by one of its creators
Compares the toroidal layouts with the well-established open layouts of the three most used platforms (Capillary, Microchip, and Slab) Provides solutions to many of the experimental issues arising in electromigration techniques and discusses the voltage distributors and detectors that are compatible with the toroidal layouts Richly illustrated with a large number of useful equations showing the relationships between important operational parameters and the performance indicators
is aimed at method developers and separation scientists working in clinical analysis, and food analysis, as well as those in pharmacology, disease biomarker applications, and nucleic acid analysis using the Capillary, Microchip, or slab Platform. It will also benefit undergraduate and graduate students of inorganic analytical chemistry, organic analytical chemistry, bioanalysis, pharmaceutical sciences, clinical sciences, and food analysis.


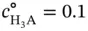 M.
M.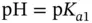 (3.13),
(3.13), 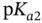 (4.76), and
(4.76), and 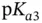 (6.39). On the other hand, Figure 1.5allows the visualization of the concentrations of extremely diluted species.
(6.39). On the other hand, Figure 1.5allows the visualization of the concentrations of extremely diluted species.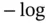 ” operator to them, produces the following:
” operator to them, produces the following: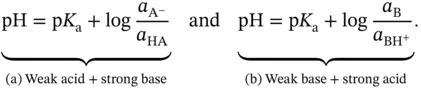
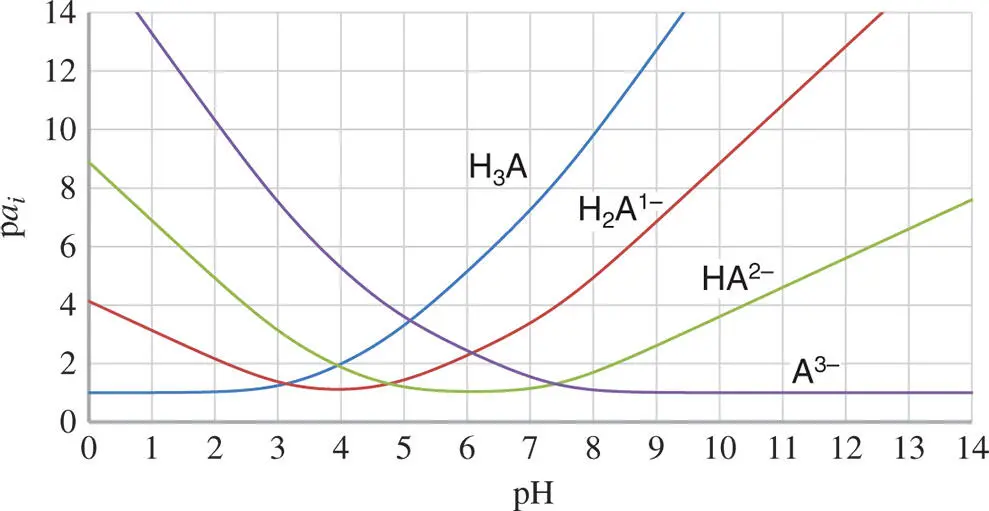
 –pH diagram of citric acid species in an aqueous solution. The conditions are identical to those used in Figure 1.4. Here, however, the concentration of the species can be seen for broader ranges.
–pH diagram of citric acid species in an aqueous solution. The conditions are identical to those used in Figure 1.4. Here, however, the concentration of the species can be seen for broader ranges.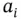 can be replaced by
can be replaced by 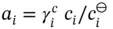 to give
to give 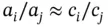 . Therefore:
. Therefore: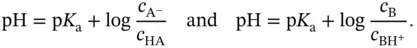
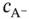 will be negligible if compared to
will be negligible if compared to 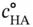 (amount of initial weak acid) before the addition of the strong base. This comes from the definition of a weak acid. Therefore, the addition of
(amount of initial weak acid) before the addition of the strong base. This comes from the definition of a weak acid. Therefore, the addition of 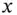 mol of a strong base to a solution containing
mol of a strong base to a solution containing 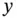 mol of a weak acid will result in
mol of a weak acid will result in 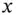 mol of A −ions, and
mol of A −ions, and 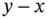 mol of HA molecules will be left in the solution. The resulting pH will then be given by:
mol of HA molecules will be left in the solution. The resulting pH will then be given by: 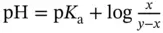 , with the condition that
, with the condition that 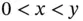 . On the other hand, if the weak acid is not so weak (or the weak base is not so weak) then the final pH will be slightly lower (or higher) than predicted. This occurs because extra A −ions, and consequently some H +ions, come from the self-ionization of the acid itself and not just from the added strong base (or strong acid).
. On the other hand, if the weak acid is not so weak (or the weak base is not so weak) then the final pH will be slightly lower (or higher) than predicted. This occurs because extra A −ions, and consequently some H +ions, come from the self-ionization of the acid itself and not just from the added strong base (or strong acid).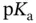 . The maximum buffer capacity of a multiprotic acid can be visualized by plotting its buffer capacity against pH (also shown in Section 1.1.12). Finally, equation 1.17can be arranged into the more useful format:
. The maximum buffer capacity of a multiprotic acid can be visualized by plotting its buffer capacity against pH (also shown in Section 1.1.12). Finally, equation 1.17can be arranged into the more useful format: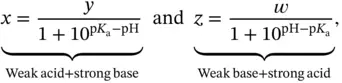
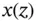 are the number of moles of a strong base (strong acid) that must be added to a solution containing
are the number of moles of a strong base (strong acid) that must be added to a solution containing 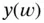 moles of a weak acid (weak base), characterized by
moles of a weak acid (weak base), characterized by 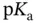 , to obtain a final solution with the desired pH. This can then be diluted until the desired concentration is achieved. The ideal situation is to have:
, to obtain a final solution with the desired pH. This can then be diluted until the desired concentration is achieved. The ideal situation is to have: 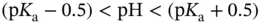 , as shown in Section 1.1.12.
, as shown in Section 1.1.12.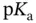 values are separated from each other by at least two units. Examples of such molecules include most proteinogenic amino acids and linear peptides without ionizable lateral chain residues. Within some pH ranges, however, if the
values are separated from each other by at least two units. Examples of such molecules include most proteinogenic amino acids and linear peptides without ionizable lateral chain residues. Within some pH ranges, however, if the 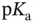 values are less than two units apart then two or more anions (or cations) from the same acid (or base) will start to coexist at concentration of more than 1% of the total molar concentration of the initial acid (or base). In this case the idea of separately visualizing conjugated acids and bases becomes a little bit confusing as the same species may start to play both roles. This is well demonstrated within the c–p and p–p diagrams. Luckily, buffer capacity (see Section 1.1.12) can still be calculated and measured under these circumstances (
values are less than two units apart then two or more anions (or cations) from the same acid (or base) will start to coexist at concentration of more than 1% of the total molar concentration of the initial acid (or base). In this case the idea of separately visualizing conjugated acids and bases becomes a little bit confusing as the same species may start to play both roles. This is well demonstrated within the c–p and p–p diagrams. Luckily, buffer capacity (see Section 1.1.12) can still be calculated and measured under these circumstances ( 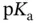 values, which are less than two units apart from each other).
values, which are less than two units apart from each other).










