But NEFAs would not be a good form in which to store lipid fuels in any quantity. Their amphipathic nature means that they aggregate in micelles (small groups of molecules, formed with their tails together and their heads facing the aqueous environment); they would not easily aggregate in a very condensed form for storage. They would also disrupt structural lipids such as those found in membranes. Triacylglycerols, on the other hand, aggregate readily; these hydrophobic molecules form uniform lipid droplets from which water is completely excluded, and which are an extremely efficient form in which to store energy (in terms of kJ stored per gram weight). This is illustrated in Figure 1.10. Thus, triacylglycerols are the form in which fat is mostly stored in the human body, and indeed in the bodies of other organisms; hence they are the major form of fat in food. NEFAs, on the other hand, are the form in which lipid energy is transported in a highly regulated manner from storage depots to sites of utilisation and oxidation.

Figure 1.10 Comparison of fat and carbohydrate as fuel sources. Raw potatoes (right) are hydrated to almost exactly the same extent as glycogen in mammalian cells. Olive oil (left) is similar to the fat stored in droplets in mature human adipocytes. The potatoes (1.05 kg) and olive oil (90 g) here each provide 3.3 MJ on oxidation. This emphasises the advantage of storing most of our energy in the body as triacylglycerol rather than as glycogen.
Proteins are chains of amino acids linked through peptide bonds. Individual proteins are distinguished by the number and order of amino acids in the chain – the sequence, or primary structure. Within its normal environment, the chain of amino acids will assume a folded, three-dimensional shape, representing the secondary structure (local folding into α-helix and β-sheet) and tertiary structure (folding of the complete chain on itself). Two or more such folded peptide chains may then aggregate (quaternary structure) to form a complete enzyme or other functional protein.
In terms of energy metabolism, the first aspect we shall consider is not how this beautiful and complex arrangement is brought about; we shall consider how it is destroyed. Protein in food is usually denatured (its higher-order structures disrupted) by cooking or other treatment, and then within the intestinal tract the disrupted chains are broken down to short lengths of amino acids before absorption into the bloodstream. Within the bloodstream and within tissues we shall be concerned with the transport and distribution of individual amino acids. These are mostly sufficiently water soluble to circulate freely in the aqueous environment of the plasma. Only tryptophan is sufficiently hydrophobic to require a transporter; it is bound loosely (like the NEFAs) to albumin. Amino acids, not surprisingly, do not cross cell membranes by simple diffusion; there are specific transporters, carrying particular groups of amino acids ( Chapter 2, Table 2.2).
Protein is often considered as the structural material of the body, although it should not be thought of as the only structural material; it can only assume this function because of the complex arrangements of other cellular constituents, especially phospholipids forming cell membranes. Nevertheless, apart from water, protein is the largest single component in terms of mass of most tissues. 1Within the body, the majority of protein is present in the skeletal muscles, mainly because of their sheer weight (around 40% of the body weight) but also because each muscle cell is well packed with the proteins (actin and myosin) which constitute the contractile apparatus. But it is important to remember that most proteins act in an aqueous environment and are, therefore, associated with water. This is relevant if we consider the body’s protein reserves as a form of stored chemical energy. Since protein is associated with water, it suffers the same drawback as a form of energy storage as does glycogen; with every gram of protein are associated about 3g of water. It is not an energy-dense storage medium. Further, although protein undoubtedly represents a large source of energy that is drawn upon during starvation, it should be remembered that there is, in animals, no specific storage form of protein; all proteins have some function other than storage of energy. Thus, utilisation of protein as an energy source involves loss of the substance of the body. In evolutionary terms we might expect that this will be minimised (i.e. the use of the specific storage compounds glycogen and triacylglycerol will be favoured) and, as we shall see in later chapters, this is exactly the case.
The monomers from which proteins are made, amino acids, have important chemical characteristics which endow them with the properties that make them ideal for assembling into a peptide chain. They comprise a central (‘α’) carbon, with characteristic chemical groups attached to its four valencies: an amino (NH 2) group, a carboxyl (COOH) group, a hydrogen atom, and finally a variable (‘R’) group which defines the actual amino acid species (for example, if R = a methyl group, CH 3, then the resulting amino acid is alanine), illustrated in Figure 1.11. At acid pH, the amino group is ionised (NH 3 +) whilst at alkaline pH the carboxyl group is ionised (COO −); at physiological pH (7.4) the amino acid is present as a Zwitter ion (both amino and carboxyl groups ionised). Proteins are assembled by adjacent amino acids forming a peptide bond between the carboxyl group of one and the amino group of another. This always leaves a terminal amino group and a terminal carboxyl group on any protein, hence all proteins act as buffers, able to gain or lose a proton. The carbon ‘backbone’ of individual amino acids is relatively energy rich and can be oxidised to yield energy once the amino group has been removed.
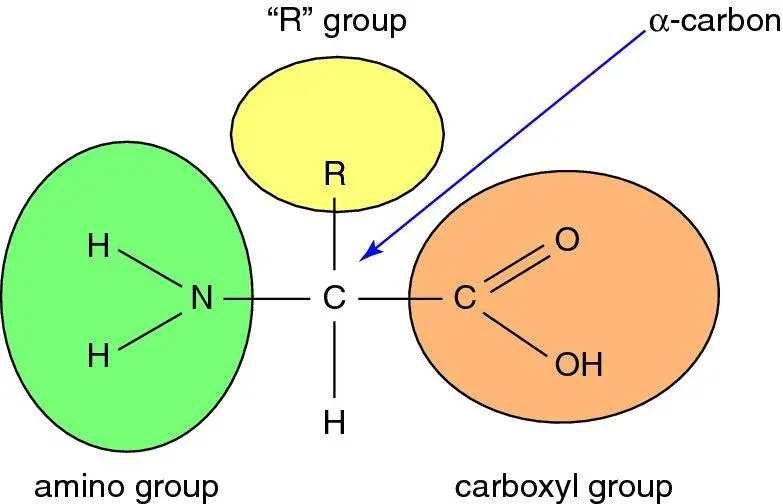
Figure 1.11 Structure of an amino acid. At physiological pH (7.4) the carboxyl group is ionised to COO −and the amino group to NH 3 +. The nature of the ‘R’ group, or side-chain , defines the particular amino acid: the 20 different amino acids which constitute proteins each have a different R group.
1.3 General overview of metabolism
1.3.1 Human metabolic pathways
The body requires energy for chemical and mechanical work in order to maintain homeostasis; functions include maintenance of ionic gradients, transport, biosynthesis, heat generation and muscle contraction. Metabolism describes the series of biochemical reactions which provide the body with the energy it requires to maintain these biological functions. This energy must ultimately be derived from food, and is sourced from three groups of energy-rich substrates: carbohydrates, lipids, and amino acids (proteins). Multiple groups are utilised because they all have chemical and thermodynamic advantages and disadvantages, and together they provide energy under widely varying conditions and demands. All three nutrient groups exist in large, energy-rich macromolecular storage forms, discussed further in Chapter 7; they are all related to daily fluxes of energy substrates in the body.
For energy mobilisation these are sequentially broken down into less energy-rich metabolites, the energy liberated being captured by intermediary reduction-oxidation molecules which carry the energy to a common pathway of oxidation linked to the phosphorylation of ADP to ATP. Hence, the energy is used to synthesise ATP, the common energy carrier to which most energy- requiring biological processes are linked. At a whole-body level this process is termed ‘catabolism’ (from the Greek: κατα ( kato ) – ‘down’ and βαλλω ( ballo ) – ‘throw’). Conversely, in energy-rich states when energy intake exceeds expenditure, these metabolic pathways can be reversed, whereby ingested nutrients from all three groups are assembled into large storage macromolecules (‘anabolism’; again, from the Greek: ανα [ ana ] – ‘up’). The process of assembling excess energy-rich substrate precursors into complex energy storage molecules is termed anabolism , whilst processes converting substrates into energy-poor end-products to mobilise biologically usable energy, are termed catabolism ( Figure 1.12and Box 1.4). Imbalance of these pathways leads to cachexia (wasting) or obesity, with implications for both energy provision and health. Tissues have specialised metabolic functions – e.g. adipose tissue stores energy, muscle oxidises substrate, lactating mammary gland exports substrate. The liver is a metabolic ‘transformer’ that regulates substrate supply between tissues, and pancreas is the principal afferent detector, and signaller, of nutritional status.
Читать дальше














