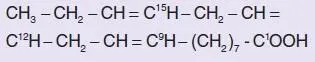Fatty acids are the building blocks of lipids, analogous to the monosaccharides. The fatty acids important in metabolism are mostly unbranched, long-chain (12 carbon atoms or more) carboxylic acids with an even number of carbon atoms. They may contain no double bonds, in which case they are referred to as saturated fatty acids , one double bond ( mono-unsaturated fatty acids ), or several double bonds – the polyunsaturated fatty acids . Many individual fatty acids are named, like monosaccharides, according to the source from which they were first isolated. Thus, lauric acid (C12, saturated) comes from the laurel tree, myristic acid (C14, saturated) from the Myristica or nutmeg genus, palmitic acid (C16, saturated) from palm oil, and stearic acid (C18, saturated) from suet, or hard fat (Greek στέαρ [ steatos ]). Oleic acid (C18, mono-unsaturated) comes from the olive (from Latin: olea , olive, or oleum , oil). Linoleic acid (C18 with two double bonds) is a polyunsaturated acid common in certain vegetable oils; it is obtained from linseed (from the Latin linum for flax and oleum for oil).
The fatty acids mostly found in the diet have some common characteristics. They are composed of even numbers of carbon atoms, and the most abundant have 16 or 18 carbon atoms. There are three major series or families of fatty acids, grouped according to the distribution of their double bonds ( Box 1.3).
Box 1.3 The structures and interrelationships of fatty acids
In the orthodox nomenclature, the position of double bonds is counted from the carboxyl end. Thus, α-linolenic acid (18 carbons, 3 double bonds) may be represented as cis -9,12, 15-18:3, and its structure is:
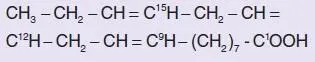
(where the superscripts denote the numbering of carbon atoms from the carboxyl end). However, this is also known as an n- 3 (or sometimes as an ω-3) fatty acid, since its first double bond counting from the non-carboxyl (ω) end is after the third carbon atom. On the latter basis, unsaturated fatty acids can be split into three main families, n- 3, n- 6, and n- 9 ( Table 1.3.1).
The saturated fatty acids can be synthesised within the body. In addition, many tissues possess the desaturase enzymes to form cis -6 or cis -9 double bonds, and to elongate the fatty acid chain ( elongases ) by addition of two-carbon units at the carboxyl end. (These steps are covered in more detail in Box 5.4) But these processes do not alter the position of the double bonds relative to the ω end, so fatty acids cannot be converted from one family to another: an n- 3 fatty acid (for instance) remains an n- 3 fatty acid. Oleic acid ( cis -9-18:1, n-9 family) can be synthesised in the human body, but we cannot form n-6 or n-3 fatty acids. Since the body has a need for fatty acids of these families, they must be supplied in the diet (in small quantities). The parent members of these families that need to be supplied in the diet are linoleic acid for the n- 6 family and α-linolenic acid for the n- 3 family. These are known as essential fatty acids . They can be converted into other members of the same family, although there seem to be health benefits of consumption of other members of the n- 3 family, particularly 20:5 n- 3 (eicosapentaenoic acid) and 22:6 n -3 (docosahexaenoic acid), found in high concentrations in fish oils. This is discussed further in Box 10.5. Some patients receiving all their nutrition intravenously have become deficient in essential fatty acids. The problem may be cured by rubbing sunflower oil into the skin!
Table 1.3.1
| Family |
Source |
Typical member |
Simplified structure |
| Saturated |
Diet or synthesis |
Myristic |
14:0 |
|
|
Palmitic |
16:0 |
|
|
Stearic |
18:0 |
| n-9 |
Diet or synthesis |
Oleic |
9-18:1 |
| n-6 |
Diet |
Linoleic |
9,12-18:2 |
| n-3 |
Diet |
α-linolenic |
9,12,15-18:3 |
Differences in the metabolism of the different fatty acids are not very important from the point of view of their roles as fuels for energy metabolism. When considering the release, transport and uptake of fatty acids (not part of triacylglycerols), the term non-esterified fatty acids (NEFAs) will therefore be used without reference to particular molecular species. In a later section (Box 10.5) some differences in their effects on the serum cholesterol concentration and propensity to heart disease will be discussed.
It will be seen from Figures 1.4and 1.9that saturated fatty acids, such as palmitic (16:0), have a natural tendency to fit together in nice orderly arrays. The unsaturated fatty acids, on the other hand, have less regular shapes ( Figure 1.9). This is reflected in the melting points of the corresponding triacylglycerols – saturated fats, such as beef suet with a high content of stearic acid (18:0), are relatively solid at room temperature, whereas unsaturated fats, such as olive oil, are liquid. This feature may have an important role in metabolic regulation, although its exact significance is not yet clear. We know that cell membranes with a high content of unsaturated fatty acids in their phospholipids are more ‘fluid’ than those with more saturated fatty acids. This may make them better able to regulate metabolic processes – for instance, muscle cells with a higher content of unsaturated fatty acids in their membranes respond better to the hormone insulin, probably because the response involves the movement of proteins (insulin receptors, glucose transporters) within the plane of the membrane (discussed in Boxes 3.2, 3.4, and elsewhere), and this occurs faster if the membrane is more fluid.
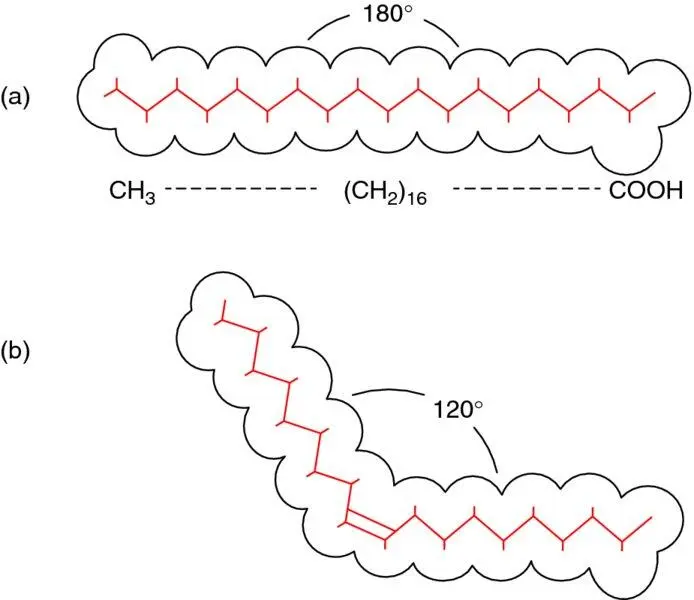
Figure 1.9 Pictures of the molecular shapes of different fatty acids. (a) saturated fatty acid, stearic acid (18:0), showing a straight chain; (b) mono-unsaturated fatty acid, oleic acid (18:1 n-9), showing a ‘bend’ in the chain at the double bond. Source: from Gurr, M. I., Harwood, J. L., Frayn, K. N., Murphy, D. J., & Michell, R. H. (2016). Lipids – Biochemistry, Biotechnology and Health . 6th edn. Oxford: Wiley.
An important feature of the fatty acids is that, as their name implies, they have within one molecule both a hydrophobic tail and a polar carboxylic acid group. Long-chain fatty acids (12 carbons and more) are almost insoluble in water. They are carried in the plasma loosely bound to the plasma protein albumin. Nevertheless, they are more water miscible than triacylglycerols, which are carried in plasma in the complex structures known as lipoproteins (discussed fully in Chapter 10). The simpler transport of NEFAs is perhaps why they serve within the body as the immediate carriers of lipid energy from the stores to the sites of utilisation and oxidation; they can be released fairly rapidly from stores when required and their delivery to tissues is regulated on a minute-to-minute basis.
Читать дальше