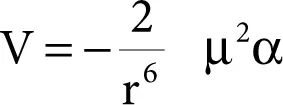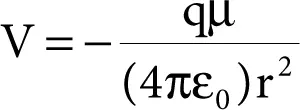ε is the permitivity of the medium
T is the temperature in Kelvin
2.3.1.3 Dipole–Induced-Dipole Interaction
In the dipole–induced-dipole interaction, the presence of the partial charges of the polar molecule causes a polarization, or distortion, of the electron distribution of the other molecule. As a result of this distortion, the second molecule acquires regions of partial positive and negative charge, and thus it becomes polar. The partial charges so formed behave just like those of a permanently polar molecule and interact favorably with their counterparts in the polar molecule that originally induced them. Hence, the two molecules cohere with a potential energy V given by
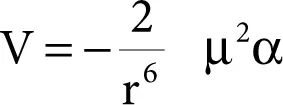
where μ is the dipole moment of the polar molecule, α is the polarizability of non-polar molecule, and r is the distance between them.
2.3.1.4 Ion–Dipole Interaction
An ion-dipoleforce is an attractive force that results from the electrostatic attraction between an ionand a neutral molecule that has a dipole.It is most commonly found in solutions. It is especially important for solutions of ioniccompounds in polar liquids. The potential energy of ion–dipole interaction is given by
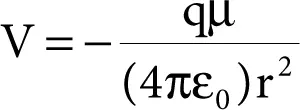
where q is the charge on the ion.
One should note that in all the above equations describing the intermolecular attractions, the denominator contains the factor r 6. Thus, the types of intermolecular interactions described above occur only at very small distances, of the order of typical atomic bond lengths (the range of non-bonding interactions is between 0.3 and 0.5 nm). For interactions to occur, therefore, the two materials must be able to make intimate contact with each other (i.e., they must be able to approach within a nanometer). This is possible if the adhesive wets the substrate efficiently. The types of interactions and the corresponding energies are given in Table 2.1.
Table 2.1 Bond types and typical bond energies [1].
| Type of interaction |
Energy (kJ/mol) |
Basis of attraction |
| Bonding |
|
|
| Ionic |
400–4000 |
Cation–anion |
| Covalent |
150–1100 |
Nuclei–shared electron pair |
| Metallic |
75–1000 |
Cations–delocalized electrons |
| Non-Bonding |
|
|
| Ion–dipole |
40–600 |
Ion charge–dipole charge |
| Hydrogen bonding |
10–40 |
Polar bond to hydrogen–dipole charge |
| Dipole–dipole |
5–25 |
Dipole charges |
| Ion–induced dipole |
3–15 |
Ion charge–polarizable electrons |
| Dipole–induced dipole |
2–10 |
Dipole charge–polarizable electrons |
| Dispersion forces |
0.1–40 |
Interaction between polarizable electrons |
This is an important intermolecular interaction specific to molecules containing an oxygen, nitrogen, or fluorine atom that is attached to a hydrogen atom. This interaction is the hydrogen bond, an interaction of the form A–H···B, where A and B are atoms of any of the three elements mentioned above and the hydrogen atom lies on a straight line between the nuclei of A and B ( Figure 2.1).
Salts like NaCl.
The acid–base character of the substrate may influence the reactivity between adhesive and substrate. A covalent bond involves shared valence electrons ( Figure 2.1).
According to Schultz and Nardin (1994), the main adhesion theories are as follows:
1 Mechanical interlocking
2 Electronic or electrostatic theory
3 Adsorption (thermodynamic) or wetting theory
4 Diffusion theory
5 Chemical (covalent) bonding theory
6 Theory of weak boundary layers and interphases
The adsorption hypothesis, which explains that adhesion is caused by intermolecular forces such as van der Waals forces, hydrogen bonds, and electrostatic interactions, is widely considered to be the most applicable to wood–polymer adhesion [7]. However, in a porous material like wood, penetration and mechanical interlocking must also play a significant role in the bonding process.
Marra [5] described adhesive bond formation in wood-based panels as a dynamic process consisting of flow, transference, penetration, wetting, and solidification (cure).
The mechanisms outlined above are not mutually exclusive since one or more of the above mechanisms can occur simultaneously depending on the specific conditions prevailing during bonding. The hierarchical cellular characteristics of wood offer such varied conditions.
The mechanical interlocking theory has long been used to explain wood bonding [6].
The electronic or electrostatic theory has been applied to wood in finishing and coating operations, although this adhesion bonding mechanism needs more fundamental research [21]. The adsorption or wetting theory has been exhaustively studied on wood over the past 40 years [7, 8].
The diffusion theory is appropriate in wood bonding during the production of compressed fibrous materials such as hardboard. The thermoplastic matrix, namely, lignin, can soften beyond its glass-transition temperature during the thermal conditions employed during hot pressing. Under these conditions, lignin can diffuse throughout the fibrous mat and react with the furfural liberated from hemicelluloses (pentosans) and solidify due to chemical reaction and hence function as an adhesive.
Besides the diffusion and molecular interpenetration of lignin occurring during wet process in the hardboard production as mentioned above, there is also the phenomenon of diffusion of monomers/oligomers of synthetic resin adhesives such as PF or UF into the wood cells followed by subsequent polymerization. This is an important concept that speaks of monomers that penetrate at a molecular level for thermosetting adhesives [9].
While discussing on the theories of adhesion in wood, one should keep in mind the opposite process (debonding). Weak boundary layers have been identified as the cause for the premature failure of the adhesive bond. In the case of wood bonding, the theory of weak boundary layers has also been proposed and studied. The weak boundary layers can be caused as a result of the mechanical damages occurring during the machining of wood surfaces. Further, the impact of surface aging the consequent inactivating of the wood surfaces [10–12] can also be responsible for the weak boundary layer.
McBain and Hopkins [13] first proposed the concept of “mechanical adhesion” in their classical paper “On adhesives and adhesive action”.
According to McBain and Hopkins, there are two kinds of adhesion: mechanical and specific adhesion. Specific adhesion involved interaction between the adherend surface and the adhesive. This interaction might be chemical interaction or adsorption.
Читать дальше