Each subatomic particle in an atom has a charge, similar to the way opposite ends of a battery or magnet are charged: positive or negative. In an atom, the protons are positive, the neutrons are neutral (no charge), and the electrons are negative. Most atoms have the same number of protons and electrons, which means the atom itself has no charge; it’s neutral.
When an atom with only one electron in its outer shell is near an atom with seven electrons in its shell, the single electron will jump over to join and complete the almost-full shell. This action results in the first atom having one more proton than electrons and, therefore, a positive (or +1) charge. Meanwhile, the second atom has one more electron than protons and, therefore, a negative (or –1) charge. (Later in the chapter, Figure 5-4 illustrates this fact.)
 Atoms or molecules (more than one atom joined together) with positive or negative charge are called ions. The charge of the ion is determined by how the electrons in its outer shell move to and from nearby atomic shells. An atom with a positive charge is called a cation, and an atom with a negative charge is called an anion. Atoms, and even compounds, can have negative charges of 1, 2, 3, and even 4 (though 4 is rare) and positive charges up to +8. The interaction of ions with one another is one way that atoms form bonds; keep reading to find out the details.
Atoms or molecules (more than one atom joined together) with positive or negative charge are called ions. The charge of the ion is determined by how the electrons in its outer shell move to and from nearby atomic shells. An atom with a positive charge is called a cation, and an atom with a negative charge is called an anion. Atoms, and even compounds, can have negative charges of 1, 2, 3, and even 4 (though 4 is rare) and positive charges up to +8. The interaction of ions with one another is one way that atoms form bonds; keep reading to find out the details.
Very few atoms exist in nature all by themselves. Multiple atoms joined together are called molecules. Some atoms of the same element pair up with each other to form molecules. An example of this is oxygen gas, which is composed of two oxygen atoms, written as O 2. (The small 2 indicates how many atoms of oxygen are in the molecule.)
In other cases, atoms of two or more different elements combine to form a compound. The compound is held together by a chemical bond. In this section, I explain the three most common types of chemical bonding between atoms.
 How two atoms bond together is determined by the number of electrons in their outer orbital shells. For example, an atom with 13 total electrons such as aluminum (Al) will have two electrons in the first orbital shell, eight electrons in the second orbital shell, and three electrons in the outermost orbital shell. The three electrons in the outermost shell are the ones that participate in bonds with other atoms.
How two atoms bond together is determined by the number of electrons in their outer orbital shells. For example, an atom with 13 total electrons such as aluminum (Al) will have two electrons in the first orbital shell, eight electrons in the second orbital shell, and three electrons in the outermost orbital shell. The three electrons in the outermost shell are the ones that participate in bonds with other atoms.
Donating electrons (ionic bonds)
When two atoms trade electrons between their outer orbital shells, becoming a cation and an anion, they form an ionic bond. The result of an ionic bond is that the positively charged cation and negatively charged anion combine into a compound that has a neutral charge. All ionic bonds create compounds called salts. Of these compounds, you are most familiar with table salt, or NaCl. In this molecule, an atom of sodium (Na) and an atom of chlorine (Cl) have bonded together. They are held together because the single electron in the outer shell of the sodium atom has been donated to fill the outer shell of chlorine (which had only seven electrons to begin with). This bond is illustrated in Figure 5-4.
Sharing electrons (covalent bonds)
When two atoms bond together, and neither one donates or gives up an electron, they form a covalent bond. In a covalent bond the atoms share the electrons in their outer orbital shells. The sharing of electrons in covalent bonding creates a very strong bond because each atom participating in the electron share has a full outer shell and a neutral charge.
An example of a covalent bond is found in a molecule of water, H 2O. As illustrated in Figure 5-5, the two atoms of hydrogen (H) share electrons with the atom of oxygen (O).
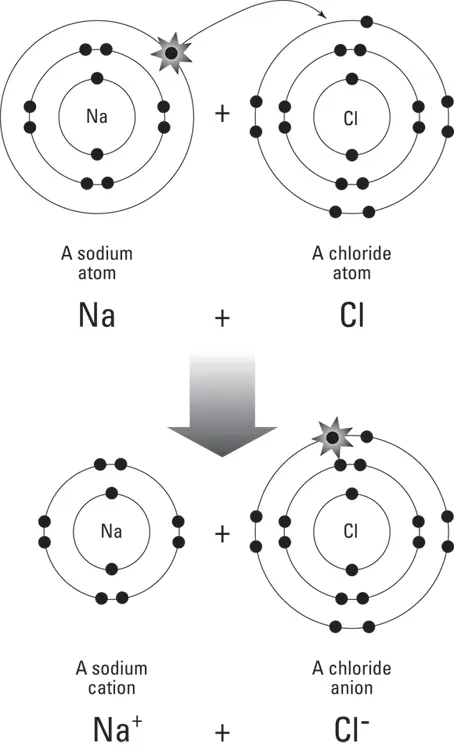
FIGURE 5-4:The ionic bond between sodium and chloride to form a molecule of NaCl.
Migrating electrons (metallic bonds)
Metallic bonds occur between atoms that have very few electrons in their outermost electron shells. Instead of donating or sharing these electrons, the electrons are released from the orbital shell and available for a nearby cluster of atoms to use. Some scientists describe the metallically bonded atoms as floating in a sea of electrons because the electrons are not specifically attached to the orbital shell of any atom in particular. The electrons in a metallic bond move freely from one atom to another. This idea is illustrated in Figure 5-6.
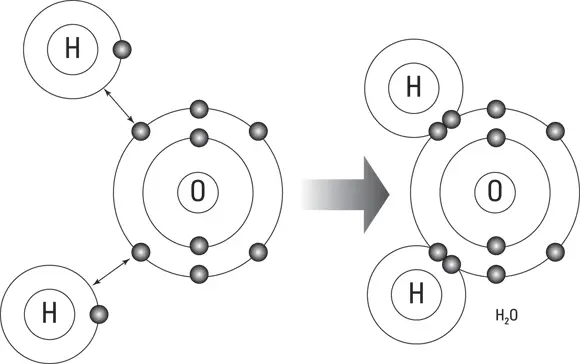
FIGURE 5-5:Covalent bonding in a water molecule.
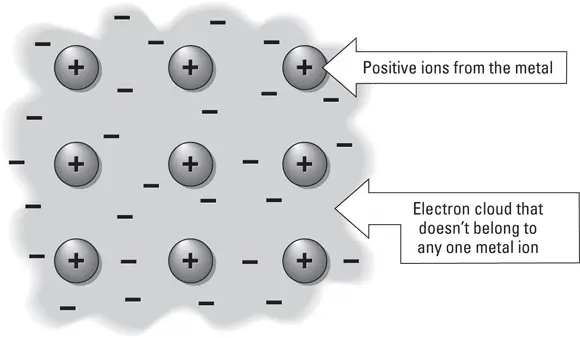
FIGURE 5-6:Metallic bonding between atoms in a sea of electrons.
 The unique nature of metallic bonds is what gives metals such as gold or silver their unique characteristics. The ability to conduct electrical current is a result of the movement of electrons. The shiny, or metallic appearance is due to the large number of freely floating electrons. And the fact that metals can be bent and molded without breaking is also a result of the movement of electrons between atoms in the metallic bond.
The unique nature of metallic bonds is what gives metals such as gold or silver their unique characteristics. The ability to conduct electrical current is a result of the movement of electrons. The shiny, or metallic appearance is due to the large number of freely floating electrons. And the fact that metals can be bent and molded without breaking is also a result of the movement of electrons between atoms in the metallic bond.
The bonding of elements to form compounds is fundamental to understanding the formation of rocks and minerals (which I describe in Chapter 6). When scientists discuss the processes of rock formation, as well as other earth processes involving chemical changes (such as weathering, described in Chapter 7), they use a shorthand of chemical formulas.
The chemical formula of a compound describes the number of different atoms of each element that are combined into a compound. For example, the chemical formula for quartz is as follows:
SiO 2
This formula indicates that one atom of silicon (Si) and two atoms of oxygen (O) have bonded together, forming the compound.
 In the case of geology, most chemical formulas describe minerals, which are solid structures built of molecules (see Chapter 6). In mineral compounds, sometimes multiple elements can fill the same spot in the mineral structure. For example, the mineral olivine has this formula:
In the case of geology, most chemical formulas describe minerals, which are solid structures built of molecules (see Chapter 6). In mineral compounds, sometimes multiple elements can fill the same spot in the mineral structure. For example, the mineral olivine has this formula:
(Mg, Fe) 2SiO 4
Two atoms of either magnesium (Mg) or iron (Fe) will combine with one atom of silicon (Si) and four atoms of oxygen (O). Either magnesium or iron can create the mineral olivine, so when you write the chemical formula, you put a parenthesis around and a comma separating the possible atoms that can form that particular chemical compound.
Конец ознакомительного фрагмента.
Текст предоставлен ООО «ЛитРес».
Читать дальше

 Atoms or molecules (more than one atom joined together) with positive or negative charge are called ions. The charge of the ion is determined by how the electrons in its outer shell move to and from nearby atomic shells. An atom with a positive charge is called a cation, and an atom with a negative charge is called an anion. Atoms, and even compounds, can have negative charges of 1, 2, 3, and even 4 (though 4 is rare) and positive charges up to +8. The interaction of ions with one another is one way that atoms form bonds; keep reading to find out the details.
Atoms or molecules (more than one atom joined together) with positive or negative charge are called ions. The charge of the ion is determined by how the electrons in its outer shell move to and from nearby atomic shells. An atom with a positive charge is called a cation, and an atom with a negative charge is called an anion. Atoms, and even compounds, can have negative charges of 1, 2, 3, and even 4 (though 4 is rare) and positive charges up to +8. The interaction of ions with one another is one way that atoms form bonds; keep reading to find out the details.


 The unique nature of metallic bonds is what gives metals such as gold or silver their unique characteristics. The ability to conduct electrical current is a result of the movement of electrons. The shiny, or metallic appearance is due to the large number of freely floating electrons. And the fact that metals can be bent and molded without breaking is also a result of the movement of electrons between atoms in the metallic bond.
The unique nature of metallic bonds is what gives metals such as gold or silver their unique characteristics. The ability to conduct electrical current is a result of the movement of electrons. The shiny, or metallic appearance is due to the large number of freely floating electrons. And the fact that metals can be bent and molded without breaking is also a result of the movement of electrons between atoms in the metallic bond.










