Take a crash course in rocks.
Chapter 5
It’s Elemental, My Dear: A Very Basic Chemistry of Elements and Compounds
IN THIS CHAPTER
 Exploring atomic structure, isotopes, and ions
Exploring atomic structure, isotopes, and ions
 Bonding atoms into molecules and compounds
Bonding atoms into molecules and compounds
 Understanding chemical formulas in earth science
Understanding chemical formulas in earth science
To understand earth processes such as the formation of rocks, it helps to understand some of the basic concepts of chemistry. The science of chemistry explores and describes the properties of substances — gas, liquid, or solid — and explains how and why different substances interact with each other.
In this chapter, I explain that all earth materials are made of atoms, and I show how these atoms interact with one another to create the observable characteristics of rocks and geologic features in the world around you.
The Smallest Matter: Atoms and Atomic Structure
 All matter is made of atoms. Every single speck of gas, liquid, or solid surrounding you is a mix of millions of atoms. An atom is the smallest bit of matter that can be measured and identified as a specific element.
All matter is made of atoms. Every single speck of gas, liquid, or solid surrounding you is a mix of millions of atoms. An atom is the smallest bit of matter that can be measured and identified as a specific element.
Atoms themselves are composed of smaller, subatomic particles called neutrons, protons, and electrons. Figure 5-1 is a diagram of atomic structure, including the location of each different subatomic particle type. Protons and neutrons are located in the nucleus at the center of the atom. In each atom, the electrons surround the nucleus, organized into orbital shells. The innermost orbital shell of any atom contains no more than two electrons; the second orbital shell contains no more than eight; and each of the outer shells, while chemically stable with eight electrons, can hold more.
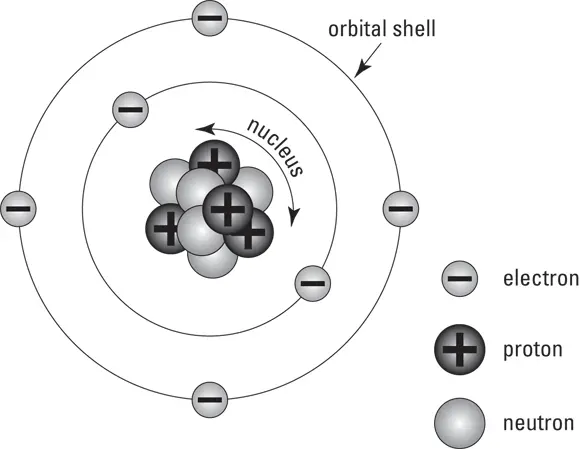
FIGURE 5-1:The parts of an atom.
 The number of protons in an atom’s nucleus determines which element the atom is. For example, an atom with six protons is an atom of the element carbon. An atom with seven protons is an atom of nitrogen.
The number of protons in an atom’s nucleus determines which element the atom is. For example, an atom with six protons is an atom of the element carbon. An atom with seven protons is an atom of nitrogen.
Getting to know the periodic table
The periodic table of elements lists the known elements in order of their atomic number, which is their number of protons. Each square on the periodic table provides you with all the information you need to know about that element and how it will interact with other elements. Figure 5-2 illustrates what the different numbers for each element on the periodic table represent:
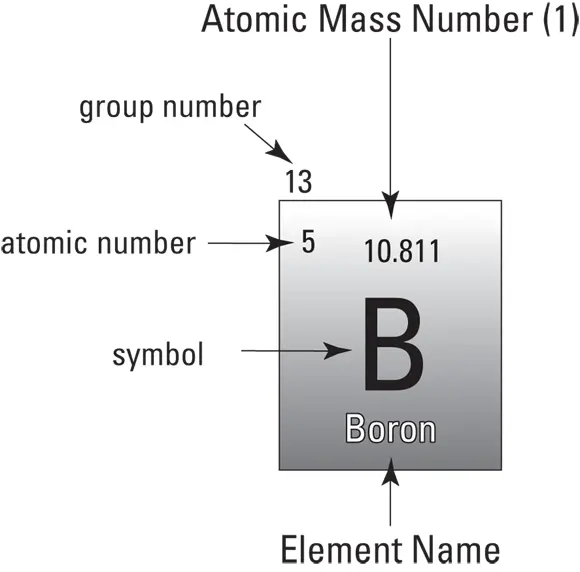
FIGURE 5-2:The parts of one square of the periodic table of elements.
Atomic mass number: The atomic mass number of an element is the total number of protons and neutrons in its nucleus.
Atomic number: The atomic number of an element is the number of protons in its nucleus.
Group number: The group number tells you how many electrons in the atom are located in the outermost orbital shell and are, therefore, available to bond it to other atoms. For example, elements in Group I have one electron in the outer electron shell, and Group II elements have two electrons in the outer electron shell. The group number for each element may help you understand why some elements, such as Magnesium (Mg) and Calcium (Ca), which are both in Group II, react in similar ways during rock formation and other geologic processes.
Symbol: The letters on the periodic table are the symbols for each element. These symbols are a shorthand so that when combinations of elements or chemical reactions are described you don’t have to write each element’s entire name. The symbols of the periodic table are the same all over the world to make it easier for scientists to communicate. In many cases, the elemental symbol is based on the name of an element in a different language and may not make sense in your native language. For example, the symbol for gold is Au because in Latin the word for gold is Aurum, which means yellow. And the symbol for tungsten is W based on its name in German: wolfram.
Element name: Some periodic tables also list the name of the element below the symbol (see Figure 5-3).
Table 5-1lists the most common elements in Earth’s crust and their approximate percentage. (This list does not represent the proportion of elements in the mantle, nor does it include the iron and nickel that are found in the earth’s core, as I describe in Chapter 4.) These elements are the ones that compose nearly all the rocks on Earth’s surface. You see them often in this book, so it’s a good idea to get familiar with their atomic symbols.
TABLE 5-1Common Elements in Earth’s Crust
| Element |
Atomic Symbol |
% of Crustal Material |
| Oxygen |
O |
46.6 |
| Silicon |
Si |
28 |
| Aluminum |
Al |
8.1 |
| Iron |
Fe |
5 |
| Calcium |
Ca |
3.6 |
| Sodium |
Na |
2.8 |
| Potassium |
K |
2.6 |
| Magnesium |
Mg |
2.1 |
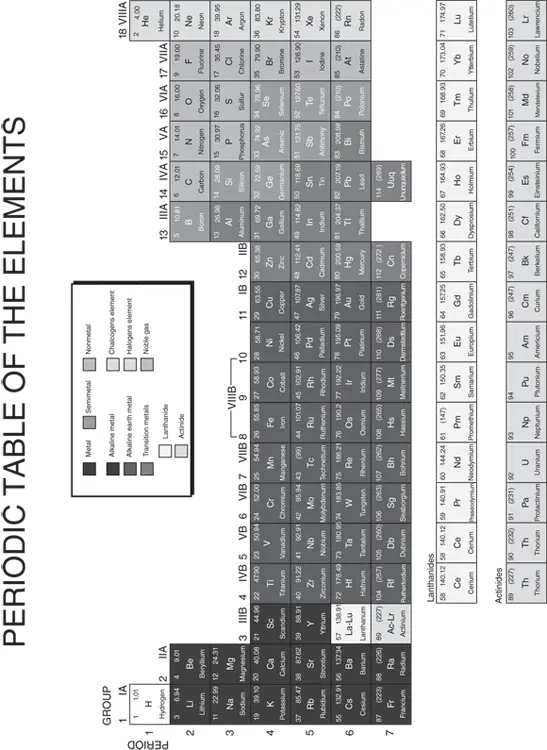
FIGURE 5-3:The periodic table of the elements.
Most elements exist as atoms of different atomic mass number, indicating different numbers of neutrons in the nucleus. As long as the number of protons stays the same (the atomic number), you have the same element, but its atomic mass changes with the addition or subtraction of neutrons. These various atoms of the same element with different atomic mass numbers are called isotopes.
Take, for example, the element carbon, which has three common isotopes:
Carbon-12 has six protons and six neutrons.
Carbon-13 has six protons and seven neutrons.
Carbon-14 has six protons and eight neutrons.
 Isotopes are very useful because although the element is the same (such as Carbon-12, Carbon-13, and Carbon-14), the heavier isotope reacts differently in chemical reactions. This means the isotopes can be counted or measured to interpret conditions of temperature or pressure when a chemical reaction occurred in the past. Also, some isotopes change or decay over time at a measurable and constant rate, which makes them useful for measuring time. You find details about how isotopes are used to determine the age of rocks in later chapters.
Isotopes are very useful because although the element is the same (such as Carbon-12, Carbon-13, and Carbon-14), the heavier isotope reacts differently in chemical reactions. This means the isotopes can be counted or measured to interpret conditions of temperature or pressure when a chemical reaction occurred in the past. Also, some isotopes change or decay over time at a measurable and constant rate, which makes them useful for measuring time. You find details about how isotopes are used to determine the age of rocks in later chapters.
Читать дальше

 Exploring atomic structure, isotopes, and ions
Exploring atomic structure, isotopes, and ions All matter is made of atoms. Every single speck of gas, liquid, or solid surrounding you is a mix of millions of atoms. An atom is the smallest bit of matter that can be measured and identified as a specific element.
All matter is made of atoms. Every single speck of gas, liquid, or solid surrounding you is a mix of millions of atoms. An atom is the smallest bit of matter that can be measured and identified as a specific element.


 Isotopes are very useful because although the element is the same (such as Carbon-12, Carbon-13, and Carbon-14), the heavier isotope reacts differently in chemical reactions. This means the isotopes can be counted or measured to interpret conditions of temperature or pressure when a chemical reaction occurred in the past. Also, some isotopes change or decay over time at a measurable and constant rate, which makes them useful for measuring time. You find details about how isotopes are used to determine the age of rocks in later chapters.
Isotopes are very useful because although the element is the same (such as Carbon-12, Carbon-13, and Carbon-14), the heavier isotope reacts differently in chemical reactions. This means the isotopes can be counted or measured to interpret conditions of temperature or pressure when a chemical reaction occurred in the past. Also, some isotopes change or decay over time at a measurable and constant rate, which makes them useful for measuring time. You find details about how isotopes are used to determine the age of rocks in later chapters.










