The injected volume of a gas sample is typically less than 0.25 mL.
The number of molecules in a fixed gas volume increases with pressure, so it's necessary to maintain constant sample pressure for each injection. Therefore, most PGCs block and bleed the sample line to allow the sample gas to come to atmospheric pressure, a technique known as atmospheric referencing. Chapter 7discusses some valve systems to achieve this.
But that still leaves the normal variation of atmospheric pressure, which is quite small, as you can see by inspecting any barometer. Discounting stormy weather, the jobsite pressure variation should not be more than about ±2 %.
In practice, atmospheric referencing works well enough for most applications. If greater precision is desired, it's best to measure local barometric pressure and adjust the measurement values to compensate for any variation found.
Some PGCs have a sensor to measure the absolute pressure of the gas sample and use an algorithm to correct for detected changes.
With liquid samples, the main challenge is to avoid gas bubbles in the injected sample as these will cause erratic measurements. To guard against bubbles, keep the pressure of a liquid sample as high as possible, consistent with the pressure rating of the sample injector valve.
A volume of liquid contains about 300 times as many molecules as an equal volume of vapor. Therefore, to inject the same number of molecules, a liquid sample volume needs to be very small, usually less than one microliter (1 μL). In such a small volume, even the smallest bubble will displace a significant amount of the sample volume and cause low measurement values.
It's easy to visualize a microliter since it's the same size as a one‐millimeter cube (1 mm 3 ). A volume of one thousand microliters is equal to one milliliter (1 mL) and to one cubic centimeter (1 cm 3 ), commonly called a cc .
Another challenge with liquid samples is getting a complete and instant vaporization without making the sample too hot, lest it start to react or decompose. The small volume is helpful, and most process liquids quickly vaporize without significant decay.
The column
The separating device
The chromatographic column is the heart of any gas chromatograph. It separates the analytes from the other sample components, and from each other, so the detector can measure them individually.
Figure 1.4pictures some typical chromatographic columns.

Figure 1.4Typical Gas Chromatographic Columns.
Source: Ohio Valley Specialty Company, Inc. Reproduced with permission.
The carrier gas carries the injected sample molecules into the column, where they touch the selected stationary phase. It's the contact with the stationary phase that causes separation. The stationary phase delays the sample molecules − some more than others − so different components end up with different transit times through the column. Each component emerges from the column after its own characteristic retention time.
A chromatographic separation takes time. In most process applications, the analysis timeis from one to ten minutes, depending on the complexity of the analyzed mixture. Some complex separations take longer.
It's important to realize that separation is just a prelude to analysis. The enormous power of the gas chromatograph comes from its ability to physically separate almost any chosen component from all other components, and then to measure it. Other analytical techniques attempt to measure the concentration of one substance in the presence of all the other substances, a goal that few accomplish well. Gas chromatographs separate the analytes first, and then measure them individually. When properly designed, a process gas chromatograph may be the only process analyzer that doesn't suffer interference from other stuff in the sample.
Of course, it's possible for two or more components to have about the same retention time in a column, so a column might not separate every component from every other component present in the sample. The task of a PGC column system is to separate the measured components from all the others. It's neither necessary nor desirable to separate everything.
The choice of separating column is always the key to a successful analysis. In practice, it's difficult to achieve the desired separation using just one column, so process gas chromatographs usually employ multiple columns to achieve the necessary separation in the shortest possible time.
With multiple columns, certain partially separated components from one column must flow into another column to achieve the desired separation. To divert flows between columns, an online gas chromatograph typically uses one or more column valvesthat are usually similar in design to its sample injection valve. Figure 1.5shows an example of a simple column system.
For simplicity, the figure shows a rotary valve that rotates 90° when actuated, thereby flushing later peaks to vent. Other valves have a similar function. 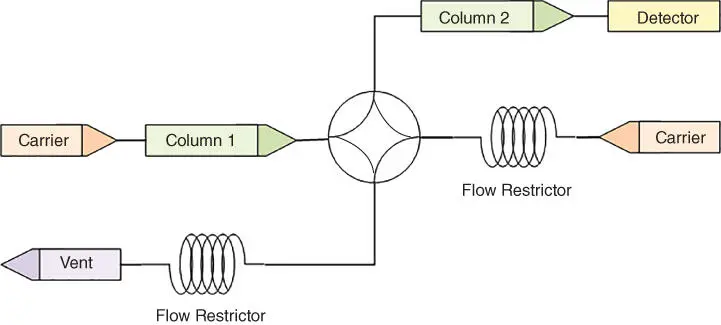
Figure 1.5A Simple Column Switching System.
Intercolumn valves must not leak. They must also have very low internal volume and smooth flow paths, lest separated components start to remix. For the same reason, a PGC typically employs 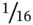 ‐inch o.d. tubing for all its internal plumbing.
‐inch o.d. tubing for all its internal plumbing.
PGCs are individually configured for a particular application. During this procedure, known as application engineering, the application engineer chooses a column system to perform the desired separation and decides on the stationary phase needed for each column. Refer to Chapter 9for a review of some standard column configurations and the function of each column.
SCI-FILE: On Column Types
Introduction to SCI‐FILEs
The more theoretical and mathematical content of the book resides in separate segments called SCI‐FILEs. These contain optional reading that may or may not be part of a course of study.
Each SCI‐FILE is a supplement to the main text that you can safely omit if not of immediate interest. Treat them as reference sources to consult when needed.
The stationary phase must be secure inside the column so it doesn't move. The packed column and the open‐tubular column differ per the method they use to anchor the stationary phase in place.
A packed columnmost often uses several meters of ⅛‐inch o.d. stainless steel tubing, although early PGCs used larger diameters, and some PGCs now employ 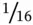 ‐inch o.d. “micropacked” columns.
‐inch o.d. “micropacked” columns.
In the traditional packed column, the packing is a granular porous solid with particles about the same size as granulated sugar. These particles pack tightly together inside the tube so that any sample molecules moving with the carrier gas are in intimate contact with them.
Читать дальше



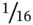 ‐inch o.d. tubing for all its internal plumbing.
‐inch o.d. tubing for all its internal plumbing. ‐inch o.d. “micropacked” columns.
‐inch o.d. “micropacked” columns.










