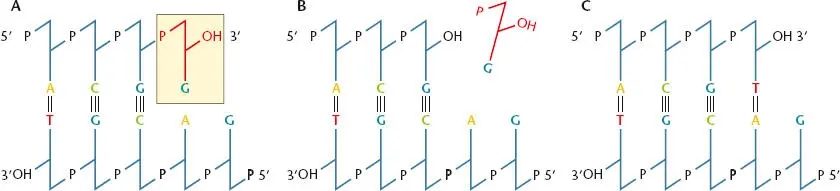
Figure 1.12 Editing function of DNA polymerase. (A)A G is mistakenly placed opposite an A while the DNA is replicating. (B and C)The DNA polymerase stops while the G is removed and replaced by a T before replication continues.
The importance of the editing functions in lowering the number of mistakes during replication may explain why DNA replication is primed by RNA rather than by DNA. When the replication of a DNA chain has just initiated, the helix may be too short for distortions in its structure to be easily recognized by the editing proteins. The mistakes may then go uncorrected. However, if the first nucleotides inserted in a growing chain are ribonucleotides rather than deoxynucleotides, an RNA primer is synthesized rather than a DNA primer. The RNA primer can be removed and resynthesized as DNA by using preexisting upstream DNA as a primer. Under these conditions, a distortion in the helix can be detected by the editing functions, and mistakes are avoided.
Another important system that safeguards the fidelity of the replication process is responsible for fixing mismatches after the growing DNA strand leaves the polymerase. In E. coli and its closest relatives, this process is guided by methylation and is termed methyl-directed mismatch repair. Related mismatch repair systems are used across all three domains of life, but the use of methylation signals is not widespread. The methyl-directed mismatch repair system is discussed in chapter 10.
Impediments to DNA Replication
While the process described above and diagrammed in Figure 1.10would suffice for pristine DNA on a template that lacked any type of physical block to the progression of the DNA replication complex, in reality, the situation in the cell is rarely this tidy. DNA polymerases frequently encounter a number of different problems. Challenges to DNA polymerases include interruptions in the DNA template, bulky adducts that cannot be replicated by DNA polymerase III, and physical blocks mediated by supercoiling and proteins bound to, or acting on, the chromosome. One extreme form of impediment to a replication fork comes from nicksin either the leading-strand or lagging-strand template DNAs in which the phosphatedeoxynucleotide chain is broken in one strand of the DNA. These nicks cause the destruction of the nick-containing arm of the replication fork, resulting in a broken chromosome and collapse of the DNA replication fork. Bacteria possess a highly efficient mechanism for priming repair of this broken end by using the broken DNA itself as a primer to reinitiate DNA replication. This process was likely the original driving force for the evolution of recombination and is described in chapter 9.
Damaged DNA and DNA Polymerase III
DNA polymerase III replicates DNA with incredibly high fidelity. Much of the fidelity of the enzyme comes from the structure of the catalytic pocket, where there is a presyntheticcheck for base pairing between the template strand and the incoming nucleotide. A side effect of this small binding pocket is the inability of the polymerase to tolerate lesionsin which chemical changes have occurred in the base, the deoxyribose sugar, or even the phosphate on the DNA. There are many mechanisms for DNA replication to continue even when a cell is grown under conditions that result in highly damaged DNA. While early work suggested that the polymerization of the leading and lagging strand was so tightly coupled that a lesion on one strand would stop the entire DNA replication fork, more recent work indicates flexibility. It is now clear that although the polymerases producing the leading strand and lagging strand are physically coupled, the two complexes can be momentarily uncoupled by leaving a singlestrand DNA gap at the point of the lesion that blocked one of the DNA polymerases. Other processes can repair these gaps, and in extreme cases, where there is extensive damage in the chromosome, these gaps initiate a DNA damage response called the SOS response (see chapter 10).
Mechanisms To Deal with Impediments on Template DNA Strands
The mechanisms used for momentarily functionally uncoupling synthesis of the two strands differ depending on whether the lesion occurs on the leading-strand or lagging-strand template. The discontinuous nature of replication on the lagging-strand template affords the opportunity to circumvent lesions that halt DNA polymerase III. Typically, DNA polymerase III is recycled onto a new DNA primer when a new RNA primer is deposited ( Figure 1.10). However, a stalled lagging-strand DNA polymerase III can also be recycled by premature release when it stalls at DNA damage ( Figure 1.13A). The single-strand DNA gap left behind is repaired by another mechanism.
Under the historical model of the function of DnaG primase, primers are placed only on the lagging-strand template. However, biochemical studies indicate that in cases where the leading-strand polymerase stalls, primase can also produce an RNA primer on the leading-strand template, allowing replication to continue but leaving a gap on this strand ( Figure 1.13B). This process of lesion skipping on the leading-strand template and the ability to utilize alternative polymerases (described below) to copy over DNA damage provide complementary mechanisms to deal with damaged DNA template strands (see Gabbai et al., Suggested Reading).
While DNA polymerase III and DNA polymerase I are important for high-fidelity DNA replication, other DNA polymerases are found in E. coli that allow replication through damaged DNA in a process known as translesion synthesis. Most translesion polymerases appear to come with a trade-off in which the ability to copy damaged DNA results in a lower fidelity of DNA replication. As expected, the expression of these polymerases is induced as a response to DNA damage in the cell. In addition to controlling the amount of translesion polymerase present in the cell, access to the DNA replication fork by polymerases other than DNA polymerase III is regulated by a process called polymerase switching, a process by which one DNA polymerase replaces a polymerase already found at the 3′ OH end of a primed DNA template ( Figure 1.13C). In E. coli , DNA polymerases II, IV, and V can be recruited to temporarily step in for DNA polymerase III at damaged DNA (more details of this system are described in chapter 10). Each of these polymerases has different attributes, ranging from fairly accurate and highly processive (DNA polymerase II) to very inaccurate and not very processive (DNA polymerases IV and V). Processivity refers to how far a DNA polymerase moves on the template before falling off.
Having multiple DNA polymerases with different properties appears to be common in all living organisms. How accurate or processive a given DNA polymerase is may also depend on the nature of the damage found in the template DNA and/or the availability of various accessory proteins. The regulation of the use of these DNA polymerases is still incompletely understood, but there appear to be highly evolved processes in which the system has been fine-tuned by the process of natural selection over a long time so that the most appropriate DNA-copying mechanism is used for each environmental challenge.
Physical Blocks to Replication Forks
Proteins bound to, or otherwise acting on, the chromosome can also stop the progression of DNA polymerase III. A programmed block to DNA replication occurs in some bacteria to terminate DNA replication within one region of the chromosome (see below). However, unintended blocks can occur in other situations, such as when DNA polymerase encounters RNA polymerase carrying out transcription (see chapter 2) or when RNA polymerase is stalled at sites of damage in the chromosome. Transcription appears to be the most significant impediment to replication, but a variety of protein complexes need to be displaced ahead of the DNA replication fork.
Читать дальше












