SEPARATING THE TWO TEMPLATE DNA STRANDS
To serve as templates for DNA replication, the two DNA strands must be separated, a task that DNA polymerase cannot perform on its own. The strands must be separated because the bases of the DNA are inside the double helix, where they are not available to pair with the incoming deoxynucleotides to direct which nucleotide will be inserted at each step. Proteins called DNA helicasesseparate the strands of DNA (see Singleton et al., Suggested Reading). Many of these proteins form a ring around one strand of DNA and propel the strand through the ring, acting as a mechanical wedge that strips the strands apart as it moves. It takes a lot of energy to separate the strands of DNA, and helicases cleave a lot of ATP for energy, forming ADP in the process. There are about 20 different helicases in E. coli , and each helicase works in only one direction, either the 3′-to-5′ or the 5′-to-3′ direction. The DnaB helicase that normally separates the strands of DNA ahead of the replication fork in E. coli is a large doughnut-shaped complex composed of six polypeptide products of the dnaB gene. It propels one strand, the template for lagging-strand DNA replication, through the center of the complex in the 5′-to-3′ direction, opening strands of DNA ahead of the replication fork ( Figure 1.10). The DnaB ring cannot load onto single-stranded DNA on its own to start a DNA replication fork; it requires the loading protein DnaC. Other helicases are discussed in later chapters in connection with recombination and repair.
Once the strands of DNA have been separated, they also must be prevented from coming back together (or from annealing to themselves if they happen to be complementary over short regions). Separation of the strands is maintained by proteins called single-strand-binding (SSB) proteins or, less frequently, helix-destabilizing proteins. They are proteins that bind preferentially to singlestranded DNA and prevent double-stranded helical DNA from reforming prematurely. Interestingly, SSB activity goes beyond this passive role. SSB is also responsible for recruiting a number of replication and repair proteins through a specific set of amino acids encoded in the very C-terminal end of SSB, allowing it to serve as an organizational hub for other processes.
PROCESSING THE TWO TEMPLATE DNA STRANDS
As discussed above, the antiparallel configuration of DNA requires that the two DNA polymerases travel in two different directions while still allowing the larger replication machine to travel in one direction down the chromosome ( Figure 1.8). This leads to fundamental differences in the natures of leading- and lagging-strand DNA replication. While replication of the leading-strand template can occur as soon as the strands are separated by the DnaB helicase, replication of the lagging-strand template is consistently reinitiated approximately every 1 to 2 kilobases (kb); this slows the process, hence the name lagging-strand synthesis. The short pieces of DNA produced from the lagging-strand template are called Okazaki fragments. Synthesis of each Okazaki fragment requires a new RNA primer about 10 to 12 nucleotides in length. In E. coli , these primers are synthesized by DnaG primase at the template sequence 3′-GTC-5′, beginning synthesis opposite the T. These RNA primers are then used to prime DNA synthesis by DNA polymerase III, which continues until it reaches the last RNA primer produced by DnaG ( Figure 1.8). Before these short pieces of DNA that are annealed to the template can be joined to make a long, continuous strand of DNA, the short RNA primers must be removed. This process is carried out by DNA polymerase I using its flap exonuclease activity to displace and cleave the RNA strand ( Figure 1.9). As DNA polymerase I displaces the RNA primer, it extends the upstream (i.e., 5′) DNA that was previously polymerized by DNA polymerase III ( Figure 1.8). Ribonuclease (RNase) H may contribute to this process under some circumstances by using its ability to degrade the RNA strand of a DNA-RNA double helix (Table 1.1). The Okazaki fragments are then joined together by DNA ligase as the replication fork moves on, as shown in Figure 1.8. By using RNA rather than DNA to prime the synthesis of new Okazaki fragments, the cell likely lowers the mistake rate of DNA replication (see below).
What actually happens at the replication fork is more complicated than is suggested by the simple picture given so far. For one thing, this picture ignores the overall topological restraints on the replicating DNA. The topologyof a molecule refers to its position in space. Because the circular DNA is very long and its strands are wrapped around each other, pulling the two strands apart introduces stress into other regions of the DNA in the form of supercoiling. If no mechanism existed to allow the two strands of DNA to rotate around each other, supercoiling would cause the chromosome to look like a telephone cord wound up on itself, an event that has been experimentally shown to eventually halt progression of the DNA replication fork. To relieve this stress, enzymes called topoisomeraseswork to help undo the supercoiling ahead of the replication fork. DNA supercoiling and topoisomerases are discussed below. The fork itself can also twist when the supercoiling that builds up ahead of the replication fork diffuses behind the replication fork, a process that twists the two new strands around one another and that is also sorted out by topoisomerases (see below).
COORDINATING REPLICATION OF THE TWO TEMPLATE STRANDS
The picture of the two strands of DNA replicating independently, as shown in Figure 1.8, does not take into consideration all of the coordination that must occur during DNA replication. The anatomy of the larger complex of replication factors remains unresolved; however, interactions among many of these components provide a hint as to how the larger complex functions ( Figure 1.10). Rather than replicating independently, the DNA polymerases that produce the leading-strand and lagging-strand DNAs are joined to each other through the τ subunits of the holoenzyme (Table 1.1). In the holoenzyme there are two τ subunits and a derivative product called γ, which is incapable of interacting with DNA polymerase. The γ and τ subunits are encoded by the same gene, dnaX . Expression of the full gene results in production of the longer τ subunit, whereas a stutter in how the protein is produced from this gene, called a “frameshift,” produces the shorter γ product. The configuration of having two τ and γ subunits may be important to ensure that only two DNA polymerase III molecules are at the replication fork, possibly facilitating the use of alternate polymerases for repair when needed (see below and Dohrmann et al., Suggested Reading).
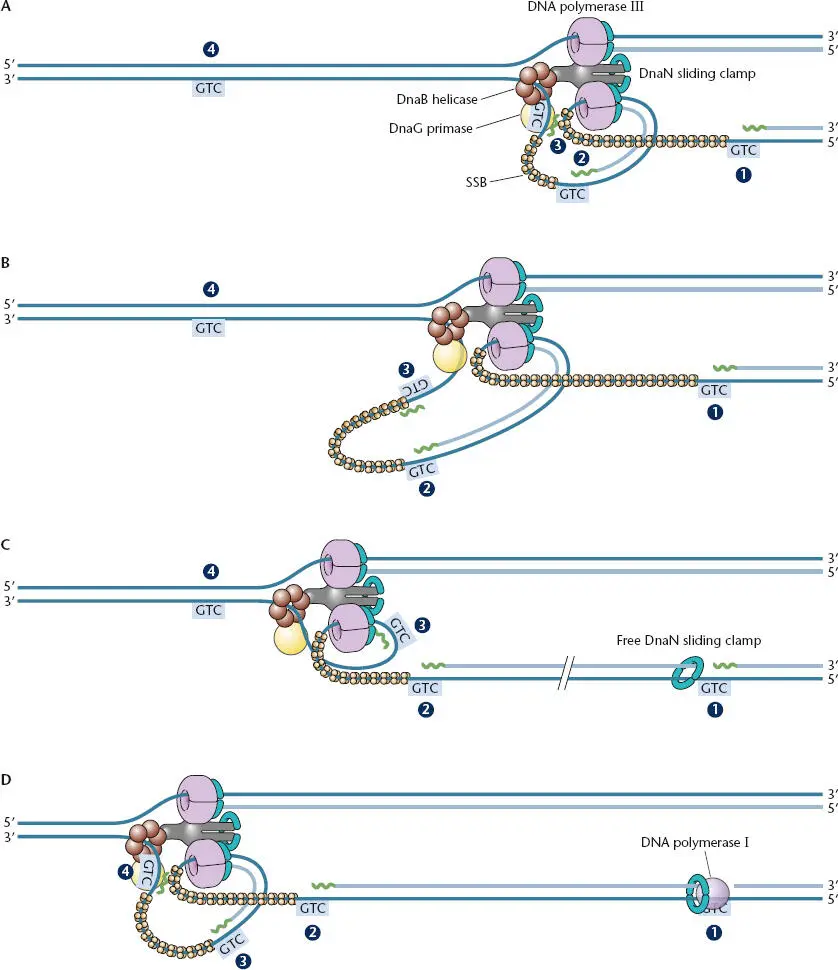
Figure 1.10 “Trombone” model for how both the leading strand and lagging strand might be simultaneously replicated at the replication fork. RNA primers are shown in green, and their initiation sites are shown as the sequence 3′-GTC-5′ boxed in blue. (A)The Pol III holoenzyme synthesizes lagging-strand DNA initiated from priming site 2 and runs into the primer at site 1. (B)The DNA strand undergoing lagging-strand replication loops out of the replication complex as the leading-strand polymerase progresses and the lagging-strand polymerase replicates toward the last Okazaki fragment. (C)Pol III has been released from the laggingstrand template at priming site 1 and has hopped ahead, leaving the old β clamps behind, and has reassembled with a new β clamp on the DNA at primer site 3 to synthesize an Okazaki fragment. Both the leading-strand and lagging-strand Pol III enzymes remain bound to each other and the helicase through interactions with τ during the release and reassembly process. (D)Pol III continues synthesis of the lagging strand from priming site 3 while Pol I is removing the primer at site 1 and replacing it with DNA. The Pol III holoenzyme hops to the primer at site 4 after reaching the primer at site 2. The primers and Okazaki fragments are not drawn to scale.
Читать дальше












