4.1.5 Mutations and Repair Mechanisms
The structure of DNA must be relatively stable and replicated almost flawlessly in order to serve as an information and inheritance carrier. DNA is a relatively stable macromolecule; however, it is liable to constant DNA damage in the body, due to internal or external causes (Table 4.4), which can manifest in mutations. Internal mechanisms are due to spontaneous depurination , deamination , oxidation, and methylationof the DNA bases; external factors include energy‐rich radiation(UV, X‐rays, and radioactivity) and mutagens. Natural mutation rates in bacteria are estimated to be 10 –5to 10 –6mutations per gene locus and generation. With eukaryotes these rates are difficult to determine but should also be in the same range.
Table 4.4 Spontaneous DNA damage in a single diploid mammalian cell within 24 hours.
| Type of DNA damage |
Number in 24 hr |
| Depurination |
18 000 |
| Depyrimidination |
600 |
| Cytosine deamination |
100 |
| 5‐Methylcytosine deamination |
10 |
| Oxidation of G to 8‐oxo G |
1500 |
| Oxidation of pyrimidines |
2000 |
| Methylation of G to 7‐methylguanosine by S‐adenosylmethionine |
6000 |
| Methylation of A to 3‐methyladenosine by S‐adenosylmethionine |
1200 |
Source: Alberts et al. (2015). Reproduced with permission of Garland Science.
Mutations where only one or a few nucleotides are exchanged are termed point mutations; other types of mutations include chromosome mutationsor rearrangementswhen larger sequence sections are cut out ( deletion) or put in ( insertionor translocation), doubled ( duplication), or oriented inversely ( inversion). If such mutations occur within a transcription unit, they are referred to as gene mutations.
In the human body, nucleotide deaminationof nucleotides also spontaneously arises with a rate of 100 deaminations per day and per cell ( Figure 4.10; Table 4.4). Cytidine is converted to uracil by deamination. If, following replication, U pairs with A instead of with G, as the original C had done, then the resulting CG pair is completely replaced with a TA pair ( Figure 4.11). Further deaminations include: adenosine to hypoxanthine (pairs with C), guanosine to xanthine, and 5‐methylcytosine to thymine (pairs with A). The purineresidues guanine and adenine can be removed spontaneously from DNA by hydrolysis ( Figure 4.10). Depurinationis considered as one of the most common spontaneous mutations and usually leads not only to transversionsbut also to the deletion of individual bases; over 18 000 purine bases are depurinated daily in every human cell. Under UV radiation(e.g. from extensive sunbathing), neighboring thymineor cytosineresidues can be activated, which then form covalently bound dimers ( dimerization). The oxidationof guanosine to 8‐oxoguanosine by oxygen radicals ( reactive oxygen species [ ROS ] )can also induce point mutations ( Figure 4.10). Therefore, ROS are assumed to play a role in processes, such as aging or cancer.
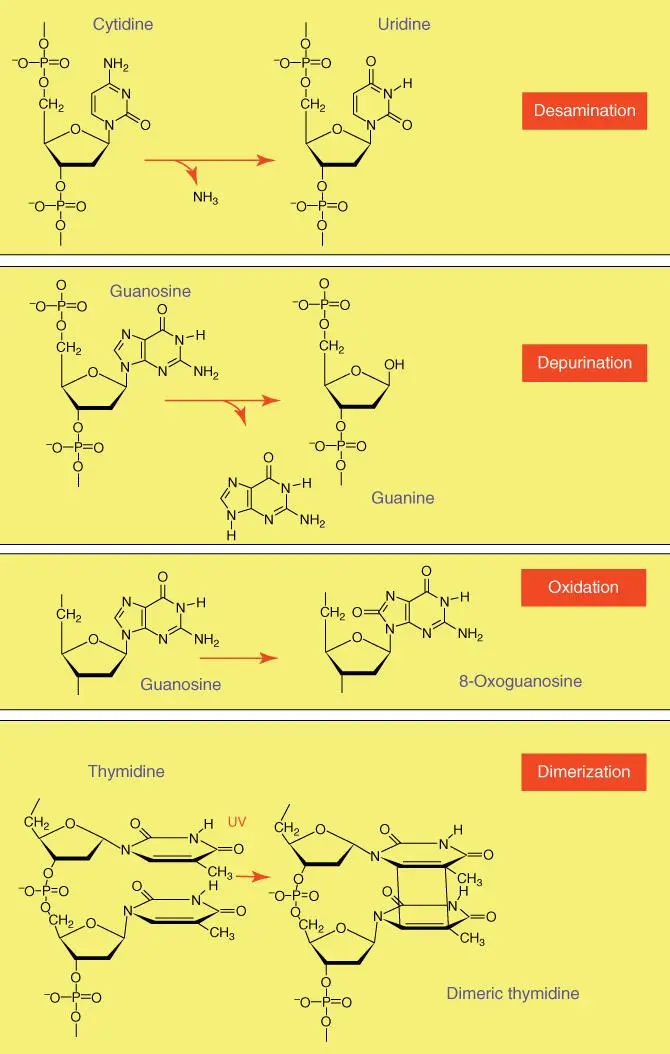
Figure 4.10 Depurination, deamination, oxidation, and dimerization as examples of major mutation mechanisms.
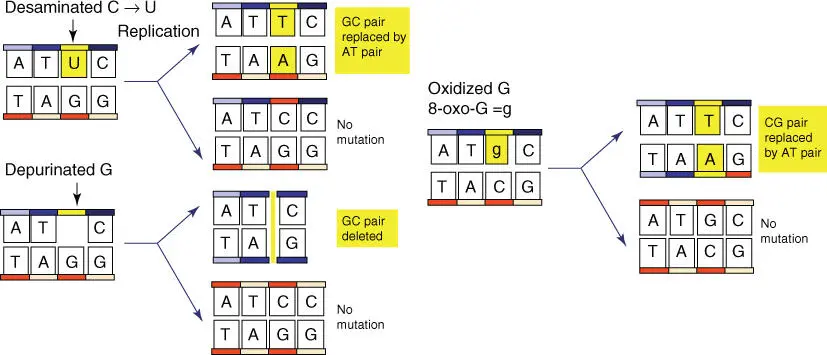
Figure 4.11 Consequences of deamination, depurination, and oxidation. Cytidine is deaminated, resulting in uracil that then pairs with adenine during replication. If a depurinated nucleotide is not repaired, DNA polymerase passes over the depurinated position during replication. A point deletion that can lead to frameshift mutations results.
In rare cases, the bases can be present in a tautomeric form( Figure 4.12). If they are selected during replication, they can lead to point mutations. G and T are normally present in the keto formand very rarely take the enol form. The amino group of A and C can in rare cases convert into an imino function. Tautomeric adenine pairs with cytosine instead of with thymine; tautomeric thymine pairs with guanine instead of with adenine and vice versa . The resulting nucleotide substitutions fall into the class of the transitions. This includes substitutions of pyrimidine bases with another pyrimidine (T to C or vice versa ) and the substitution of a purine base by another (A → G or vice versa ). Transversionsinclude the exchange of a purine base with a pyrimidine (A → C or T/G → C or T and vice versa ).
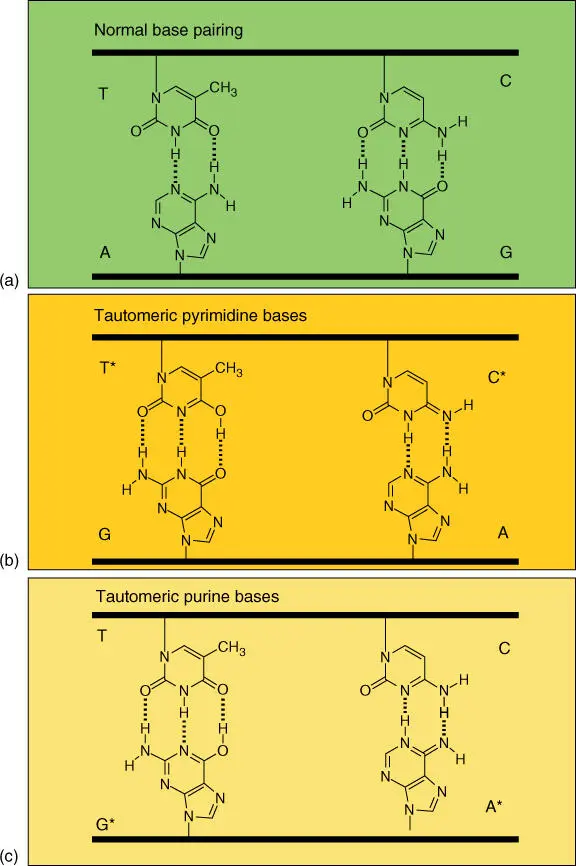
Figure 4.12 Base pairing of tautomeric DNA bases. The correct base pairings for A–T and G–C pairs are illustrated in (a). Base pairs between tautomers A, G, C, and T are shown in (b) and (c).
Most of the primary gene changes (deamination, depurination, dimerization, and oxidation) are recognized by repair enzymes(such as AP endonuclease and DNA glycosylases), cut out (as long as the second DNA strand is not also damaged), and repaired by DNA polymerase (especially translesion polymerases) and DNA ligase. In addition, alkyltransferases, photolyases, and incorrect pairing repair and recombination repair systems (see homologous recombination) are also available, which are active after replication. The double helix is also advantageous for any repair process, as genetic information is complementarily saved. Even if the information on one strand is lost, the complementary strand is still available and can be used as a template for the needed correction. In gametes, such as those of humans, thanks to the effectiveness of the repair systems, there are only 10–20 nucleotide substitutions per year in relation to the available 3.2 × 10 9bases. The significance of the repair system is easily recognized in humans who are affected by xeroderma pigmentosum, a rare autosomal recessive neurocutaneous disease, in which certain elements of the repair system that are required to repair DNA damage caused by UV radiation do not function. As a result of mutagenic UV radiation from sunlight, aside from numerous neurological and psychiatric symptoms, skin discoloration and skin cancer can occur. This can only be prevented by complete avoidance of sunlight. Table 4.5lists further diseases caused by defective repair enzymes.
Table 4.5 Genetic diseases, which are associated with defective DNA repair systems.
| Syndrome |
Phenotype |
Damaged proteins |
| MH2,3,6; MLH1; PMS2 |
Colon cancer |
Mismatch repair |
| Xeroderma pigmentosum (XP) |
Skin cancer, neurological disorders |
Excision repair |
| Cockayne syndrome |
UV sensitivity, developmental abnormalities |
Coupling of nucleotide excision repair to transcription |
| XP variant |
UV sensitivity, skin cancer |
Translesion synthesis by DNA polymerase |
| BRCA1 |
Breast and ovarian cancer |
Repair through homologous recombination |
| BRCA2 |
Breast, ovarian, and prostate cancer |
Repair through homologous recombination |
| Werner syndrome |
Premature aging, many tumors |
3‐Exonuclease, DNA helicase for DNA repair |
| Bloom syndrome |
Many tumors, stunted growth, genome instability |
DNA helicase for DNA repair |
| Fanconi anemia groups A–G |
Malformations, leukemia, genome instability |
DNA interstrand cross‐link repair |
| 46 BR‐patient |
Hypersensitivity for mutagenic substances, genome instability |
DNA ligase I |
Source: Alberts et al. (2015). Reproduced with permission of Garland Science.
Читать дальше






![Andrew Radford - Linguistics An Introduction [Second Edition]](/books/397851/andrew-radford-linguistics-an-introduction-second-thumb.webp)








