1 The acid–base catalysis. Hydrogen bonds are formed in the transition state, which stabilize the transition state (decrease the level of its free energy) to lower the activation energy (ΔGǂ). As a result, the reactivity of the substrate is enhanced and the reaction goes faster.
2 The metal–ion catalysis. The catalytic center is a metal ion which is attached to functional groups of some side chains of α‐amino acid residues of the enzyme. A coordination bond between the substrate and the metal ion is formed (or partially formed) in the transition state to stabilize the transition state and activate the substrate.
3 The covalent catalysis. A covalent bond between the substrate and the enzyme is formed (or partially formed) in the active site in the transition state to stabilize the transition state and activate the substrate.
A remarkable feature for enzymatic reactions is that the enzyme and substrate are both structurally and electronically complementary (also called key‐to‐lock model). As a result, the catalytic efficiency is extremely high. An enzymatic reaction is often 10 6–10 12times as fast as the uncatalyzed reaction [8]. In addition, most of the enzymatic reactions are highly selective and stereospecific due to the enzyme–substrate complementarity.
Many common enzymatic reactions follow mechanisms of the acid–base catalysis. The catalysis is usually concerted and achieved by proton transfer between the enzyme and substrate. The general principle for this type of catalysis can be illustrated using a generalized keto–enol tautomerization ( Fig. 1.21).
Figure 1.21a shows an uncatalyzed, concerted keto–enol tautomerization taking place via a single transition state. The transition state is greatly destabilized (with a high level in free energy) by the formation of partial electric charges in different atoms. Figure 1.21b and c show acid‐ and base‐catalyzed tautomerization, respectively. The acid H–A and base :B −represent acidic and basic functional groups, respectively, in the active sites of enzymes. In the acid catalysis ( Fig. 1.21b), a hydrogen bond is formed in the transition state on the carbonyl of the keto substrate to stabilize the partial negative charge on oxygen (via the electrostatic attraction to the partial positive charge on the HA proton). This stabilization leads to decrease in the energy level (in the free energy term) of the transition state and the activation energy (Δ G ǂ) is lowered. In the base catalysis ( Fig. 1.21c), a partial deprotonation on α‐carbon by the basic group occurs in the transition state. As a result, the partial positive charge developed on the α‐hydrogen of the keto substrate is stabilized (via the electrostatic attraction to the negative charge in the basic group B −). This stabilization leads to decrease in the free energy level of the transition state and the activation energy is lowered. In both acid‐ and base‐catalyzed tautomerization ( Fig. 1.21b and c), the formations of the enol and regeneration of the free enzymes from the transition states (TS1 and TS2) proceed via additional proton transfer steps. However, these steps are spontaneous and fast and do not affect the activation energies. The comparison of energetics for the uncatalyzed tautomerization and acid‐ and base‐catalyzed tautomerization is demonstrated in Figure 1.21d.
Very often, enzymatic catalysis can also be achieved by splitting an uncatalyzed mechanistic step with a high activation energy into multiple catalyzed microscopic steps with lower activation energies. This way of catalysis can be demonstrated by using carbonic anhydrase , the enzyme that catalyzes the reaction of carbon dioxide (CO 2) with water (H 2O) giving bicarbonate (HCO 3 −) in human blood [8]. When CO 2produced from respiration is transferred into the venous blood, it combines with water in the blood and this reaction happens. By virtue, the reaction of CO 2with H 2O is a simple inorganic chemical reaction. When it happens in the venous blood and is catalyzed by carbonic anhydrase, it becomes a biochemical reaction.
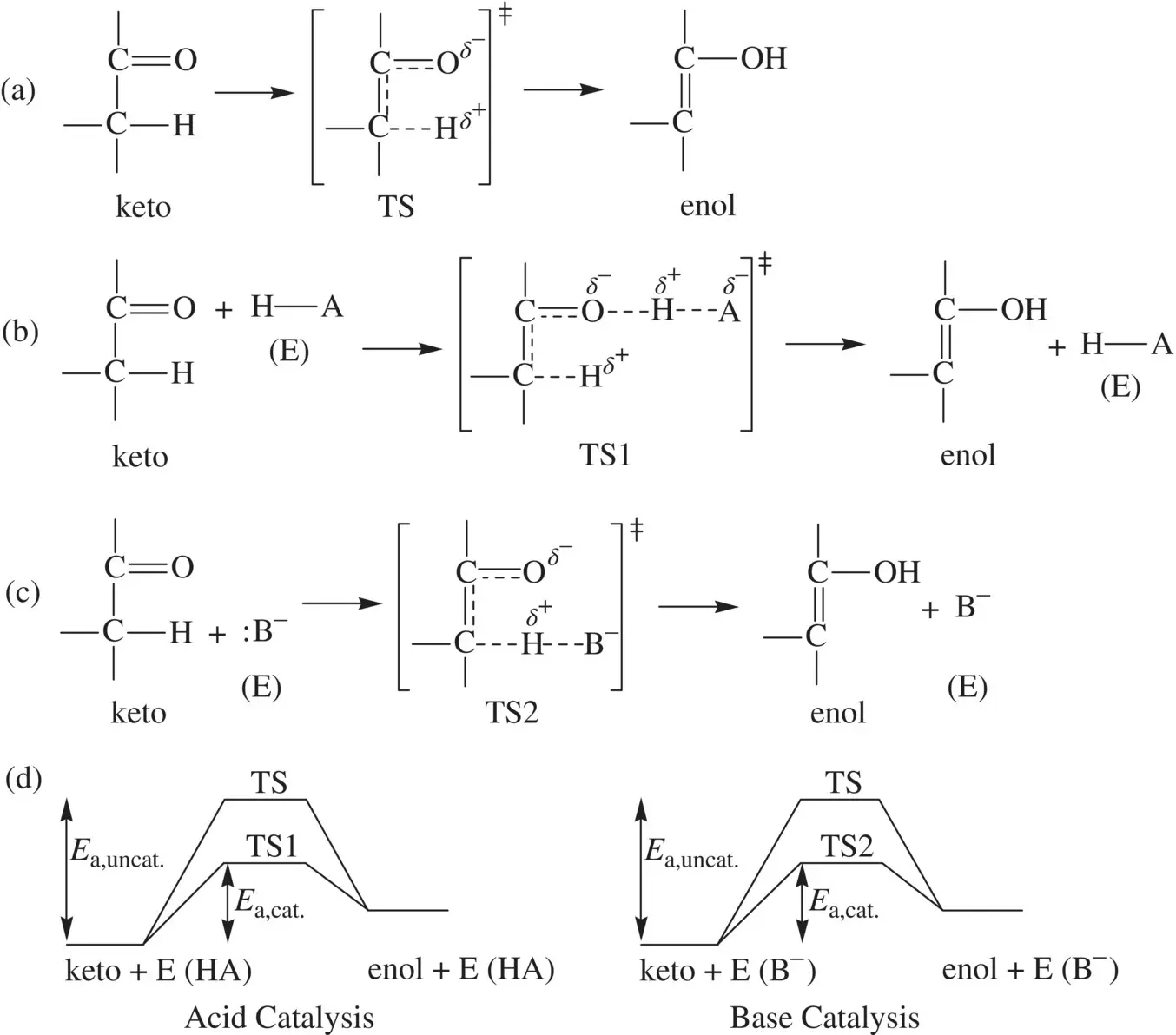
FIGURE 1.21 Acid–base catalysis for enzymatic reactions. (a) Uncatalyzed concerted keto–enol tautomerization; (b) The acid‐catalyzed mechanism for the keto–enol tautomerization; (c) The base‐catalyzed mechanism for the keto–enol tautomerization; and (d) Comparison of energetics for uncatalyzed and acid‐ or base‐catalyzed keto–enol tautomerization.
The catalytic center of carbonic anhydrase is a sp 3‐hybridized zinc ion (Zn 2+) connecting to three imidazole rings (Im) of histidine residues in the enzyme's polypeptide chain [8]. The unoccupied (vacant) sp 3orbital in Zn 2+is strongly electrophilic. The transition state (TS) of the uncatalyzed reaction of CO 2with H 2O is highly destabilized (with a high level of free energy) due to the formation of partial electric charges ( Fig. 1.22a). In the presence of carbonic anhydrase, the reaction pathway is altered such that the first step of the reaction is that H 2O (hydrogen bonded to the enzyme) coordinates to the electrophilic Zn 2+center in the enzyme to lead to the formation of a nucleophilic hydroxide (OH −) attached to Zn 2+( Fig. 1.22b) [8]. The transition state (TS1) is substantially stabilized, relative to that (TS) of the uncatalyzed reaction, by the partially formed O…Zn 2+coordination bond. Then the strongly nucleophilic OH −in the Zn 2+center attacks CO 2to bring about a nucleophilic addition with a low‐level transition state (TS2), giving HCO 3 −and regenerating a free enzyme ( Fig. 1.22b) [8]. In the in vivo reaction, the proton by‐product combines with hemoglobin in the blood. The comparison of energetics and mechanisms for the uncatalyzed and enzyme catalyzed reactions of CO 2with H 2O is demonstrated in Figure 1.23.
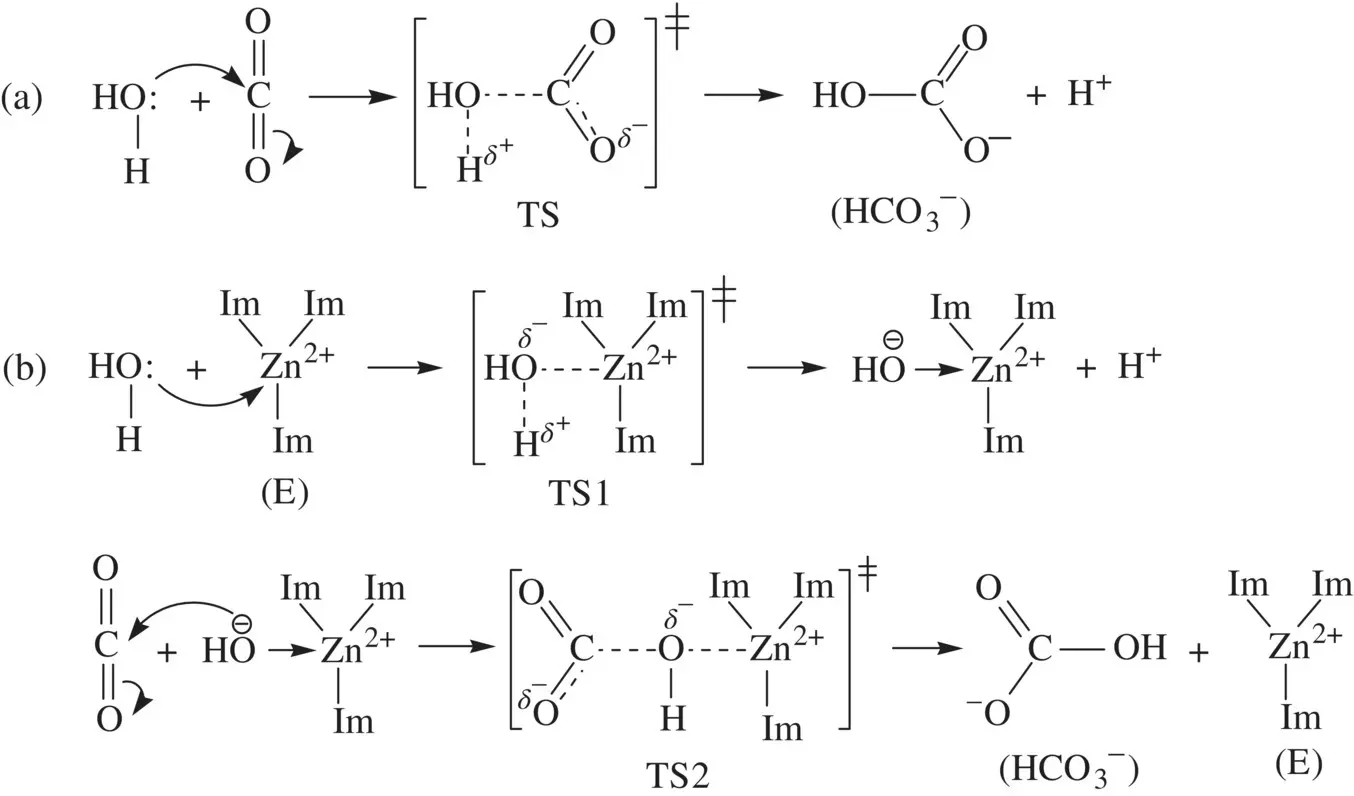
FIGURE 1.22 (a) Mechanism for the concerted reaction of H 2O and CO 2giving HCO 3 −and (b) Mechanism for the enzyme (carbonic anhydrase) catalyzed stepwise reaction of H 2O and CO 2giving HCO 3 −.
Many biochemical reactions follow some fundamental organic reaction mechanisms demonstrated in this book. Various biological applications of the mechanisms are discussed in all the individual chapters.
1.12 THE GREEN CHEMISTRY METHODOLOGY
Traditionally, synthesis of organic compounds has been performed very commonly in various organic solvents to make homogeneous reactions occur because many organic reactants are hydrophobic (water insoluble), but lipophilic (soluble in “oil”—the organic media). Organic solvents are in general hazardous substances. Utilization of large amounts of toxic organic solvents in synthetic reactions has created a big burden to the environment. The ideal green synthesis of organic compounds should avoid utilization of any solvents or only include environmentally benign solvents in the reactions, among which the most convenient, most natural, and cheapest is water. Conducting organic reactions in (or on) water will minimize the difficulty in the waste disposal, greatly reduce the pollution of the environment with hazardous materials, and ease the product work‐up and separation. This protocol has received much attention from organic chemists in the past 15 years [9, 10]. Many synthetic reactions performed in water heterogeneously have been found substantially faster than the reactions traditionally carried out in organic solvents [9–12]. Effective and efficient organic synthesis in water represents a major aspect of methodology in the current green chemistry.
Читать дальше














