Remote C-H Bond Functionalizations
Здесь есть возможность читать онлайн «Remote C-H Bond Functionalizations» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Remote C-H Bond Functionalizations
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Remote C-H Bond Functionalizations: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Remote C-H Bond Functionalizations»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Remote C—H Bond Functionalizations
Remote C—H Bond Functionalizations
Remote C-H Bond Functionalizations — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Remote C-H Bond Functionalizations», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
The representative template assisted meta‐ C–H activation is olefination via Pd(II)/Pd(0) process with the nitrile‐based directing template. Thus, a tentative catalytic cycle could be proposed for this reaction ( Scheme 2.65). The catalytic process proceeds through five major steps: C–H activation, olefin binding and alkene migratory insertion, β‐hydride elimination, reductive elimination, and re‐oxidation of the Pd‐catalyst. Computational study using density functional theory elucidated that the C–H activation step, which proceeds via a concerted metalation–deprotonation (CMD) pathway, was the rate‐ and regioselectivity‐determining step [55]. However, unlike the presentation in Scheme 2.65, it was found that the C–H activation with the nitrile‐based directing template occurred via a Pd–Ag heterodimeric transition state ( Scheme 2.66). Moreover, the nitrile directing template coordinated with Ag an end‐on fashion rather than the Pd metal center. The Pd was then linked with Ag by the bridging acetate ligand and delivered to the meta ‐C  H bond in the transition state, resulting in the observed high meta ‐selectivity. However, it is worth noting that not all the template assisted meta‐ C–H olefination required the use of silver salt, such as the meta‐ C–H olefination of benzoic acids by the Li group [28]. In addition, significant effects of distortions of the template were observed in the structural and distortion energy analysis of the transition states, which had help to devise the conformationally flexible directing template for benzoic acids by the groups of Houk and Yu in 2017 [29].
H bond in the transition state, resulting in the observed high meta ‐selectivity. However, it is worth noting that not all the template assisted meta‐ C–H olefination required the use of silver salt, such as the meta‐ C–H olefination of benzoic acids by the Li group [28]. In addition, significant effects of distortions of the template were observed in the structural and distortion energy analysis of the transition states, which had help to devise the conformationally flexible directing template for benzoic acids by the groups of Houk and Yu in 2017 [29].
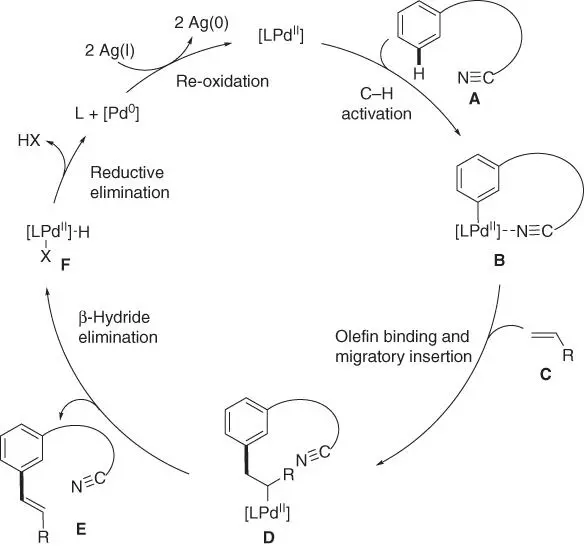
Scheme 2.65Proposed catalytic cycle for meta‐ C–H olefination.
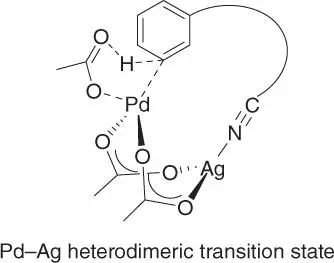
Scheme 2.66Proposed transition state through computational study.
Subsequently, Houk, Yu, Wu, and coworkers revealed the dual roles of the amino acid ligand in improving reactivity and selectivity through mass spectrometry (MS) and DFT calculations [56]. It was found that the amino acid ligand aced as both a dianionic ligand and a proton acceptor, leading to the stabilization of the monomeric Pd complexes and serving as the internal base for proton abstraction through a CMD pathway.
Besides the MPAA ligand, the HFIP solvent is another key factor for most of the template assisted meta‐ C–H activation reactions. Although the exact role of HFIP is unknown at present, Maiti and coworkers proposed that the solvent HFIP could act as a coordinating ligand in the early stage of the reaction to promote the reaction through experimental and computational investigation [40,54]. Moreover, they also found that the hydrogen‐bonding between HFIP and the pyrimidine‐based template was vital to decrease the basicity of the pyrimidine group and increase the π‐acidity of the Pd center based on nuclear magnetic resonance (NMR) studies [51].
2.4 Conclusion
Since 2012, the directing template approach has promoted a range of meta‐ selective C–H activation reactions with several classes of substrate. Three major classes of directing templates have been engineered by recognition of the geometry and distance between the directing atom and the target meta‐ C  H bonds. The number of transformation types also increases gradually since the first discovery of meta‐ C–H olefination. Despite the advances, the directing template strategy still suffers from several limitations. First, the transformation type is still limited, such as amination, fluorination, and alkynylation are not feasible at present. Thus, new protocols with possible new templates are needed. Second, it is not step‐economic to link the template with the substrate with a covalent bond. Although breakthroughs in using catalytic amount of templates have been disclosed, these protocols were limited to special substrates and suffer from limitations such as high metal catalyst loadings and high molecular weights. Therefore, the discovery of more efficient systems using template through non‐covalent interaction is highly desirable. Third, the high catalyst loading and the use of precious metal catalysts are not practical for the application of this method for large scale synthesis. The search for more effective catalytic protocols using low‐cost metal catalysts and even in a low loading is well‐worth investigating. In short, there are still many opportunities for exciting discoveries in the field of meta‐ C–H functionalization assisted by directing templates.
H bonds. The number of transformation types also increases gradually since the first discovery of meta‐ C–H olefination. Despite the advances, the directing template strategy still suffers from several limitations. First, the transformation type is still limited, such as amination, fluorination, and alkynylation are not feasible at present. Thus, new protocols with possible new templates are needed. Second, it is not step‐economic to link the template with the substrate with a covalent bond. Although breakthroughs in using catalytic amount of templates have been disclosed, these protocols were limited to special substrates and suffer from limitations such as high metal catalyst loadings and high molecular weights. Therefore, the discovery of more efficient systems using template through non‐covalent interaction is highly desirable. Third, the high catalyst loading and the use of precious metal catalysts are not practical for the application of this method for large scale synthesis. The search for more effective catalytic protocols using low‐cost metal catalysts and even in a low loading is well‐worth investigating. In short, there are still many opportunities for exciting discoveries in the field of meta‐ C–H functionalization assisted by directing templates.
Abbreviations
AcacetylAlaL‐alanineArarylBoctert‐butyloxycarbonylBnbenzylBubutylCANceric ammonium nitratecat.catalyticCMDconcerted metalation–deprotonationDCE1,2‐dichloroethaneDGdirecting templateDIH1,3‐diiodo‐5,5‐dimethylhydantoinDMFN,N‐dimethylformamideDMPU1,3‐dimethyl‐3,4,5,6‐tetrahydro‐2(1H)‐pyrimidinoneequivequivalentEDGelectron‐donating templateEWGelectron‐withdrawing templateGlyglycineHFIPhexafluoroisopropanolKIEkinetic isotope effectLligandLDAlithium diisopropylamidemmetaMemethylMPAAmono‐N‐protected amino acidNMPN‐methylpyrrolidinoneoorthopparaPhphenylPheL‐phenylalaninePinpinacolPivpivaloylPrpropylTBAtetrabutylammoniumTFAtrifluoroacetic acidTBAPF6tetrabutylammonium hexafluorophosphateTFEtrifluoroethanolTHFtetrahydrofuranXPhos2,4′,6′‐diisopropyl‐1,1′‐biphenyl‐2‐yldicyclohexylphosphine
References
1 1 Truong, T. and Daugulis, O. (2012). Angew. Chem. Int. Ed. 51: 11677.
2 2 Julia‐Hernandez, F., Simonetti, M., and Larrosa, I. (2013). Angew. Chem. Int. Ed. 52: 11458.
3 3 Ackermann, L. and Li, J. (2015). Nat. Chem. 7: 686.
4 4 Frost, C.G. and Paterson, A.J. (2015). ACS Cent. Sci. 1: 418.
5 5 Li, J., De Sarkar, S., and Ackermann, L. (2016). Top. Organomet. Chem. 55: 217.
6 6 Yang, J. (2015). Org. Biomol. Chem. 13: 1930.
7 7 Chattopadhyay, B. and Bisht, R. (2016). Synlett 27: 2043.
8 8 Dey, A., Agasti, S., and Maiti, D. (2016). Org. Biomol. Chem. 14: 5440.
9 9 Ghosh, M. and De Sarkar, S. (2018). Asian J. Org. Chem. 7: 1236.
10 10 Dey, A., Sinha, S.K., Achar, T.K., and Maiti, D. (2019). Angew. Chem. Int. Ed. 58: 10820.
11 11 Leow, D., Li, G., Mei, T.S., and Yu, J.‐Q. (2012). Nature 486: 518.
12 12 Zhang, Z., Tanaka, K., and Yu, J.‐Q. (2017). Nature 543: 538.
13 13 Achar, T.K., Ramakrishna, K., Pal, T. et al. (2018). Chem. Eur. J. 24: 17906.
14 14 Modak, A., Mondal, A., Watile, R. et al. (2016). Chem. Commun. 52: 13916.
15 15 Li, S., Wang, H., Weng, Y., and Li, G. (2019). Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.201910691.
16 16 Xu, H.‐J., Lu, Y., Farmer, M.E. et al. (2017). J. Am. Chem. Soc. 139: 2200.
17 17 Xu, H.‐J., Kang, Y.‐S., Shi, H. et al. (2019). J. Am. Chem. Soc. 141: 76.
18 18 Wan, L., Dastbaravardeh, N., Li, G., and Yu, J.‐Q. (2013). J. Am. Chem. Soc. 135: 18056.
19 19 Xu, H., Liu, M., Li, L.J. et al. (2019). Org. Lett. 21: 4887.
20 20 Bera, M., Modak, A., Patra, T. et al. (2014). Org. Lett. 16: 5760.
21 21 Deng, Y. and Yu, J.‐Q. (2015). Angew. Chem. Int. Ed. 54: 888.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Remote C-H Bond Functionalizations»
Представляем Вашему вниманию похожие книги на «Remote C-H Bond Functionalizations» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Remote C-H Bond Functionalizations» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












