Remote C-H Bond Functionalizations
Здесь есть возможность читать онлайн «Remote C-H Bond Functionalizations» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Remote C-H Bond Functionalizations
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Remote C-H Bond Functionalizations: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Remote C-H Bond Functionalizations»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Remote C—H Bond Functionalizations
Remote C—H Bond Functionalizations
Remote C-H Bond Functionalizations — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Remote C-H Bond Functionalizations», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
In 2019, Paton, Maiti, and coworkers developed the Palladium(II)‐catalyzed meta ‐selective C–H allylation of alcohol derivatives using synthetically inert acyclic internal olefins as allylic surrogates, under the conditions discussed earlier for benzylsulfonyl esters ( Scheme 2.57) [27]. Experimental and computational studies implied that the pyrimidine‐based directing group was crucial this meta ‐selective C–H activation in determining the product selectivities. Notably, besides phenylethyl alcohols, alcohol derivatives with longer chain homologues could also be viable in this transformation.
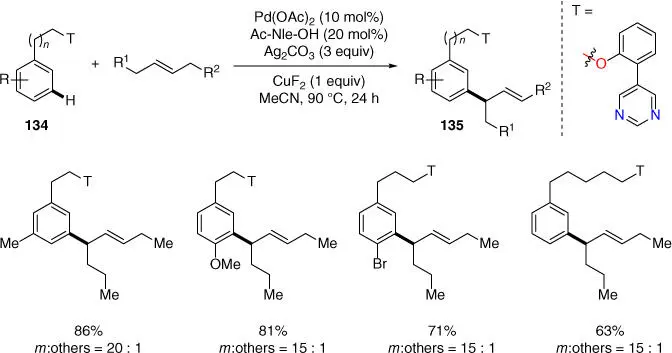
Scheme 2.57 meta ‐C–H allylation of alcohol derivatives.
Source: Modified from Achar et al. [27].
Recently, Wang, Kong, and coworkers developed the palladium‐catalyzed meta ‐olefination of arene‐tethered diols assisted by a pyrimidine‐based template ( Scheme 2.58) [52]. It was demonstrated this method could be used for the facile synthesis of various diol‐based natural products such as coumarins. Moreover, removal of the template was easily realized by hydrolysis under acidic conditions.

Scheme 2.58 meta ‐C–H olefination of arene‐tethered diols.
Source: Modified from Fang et al. [52].
Finally, Yu, Dai, and coworkers also achieved meta‐ C–H deuteration of benzyl and phenyl ethyl alcohols with a pyridine‐based template using easily available deuterium source such as deuterated acetic acid ( Scheme 2.59) [19]. The template was linked to the substrate through a practical ester linkage that could be easily installed and cleaved. With high levels of D‐incorporation at the meta ‐positions, this approach is potentially useful since deuterium‐labeled compounds are important for mechanistic and metabolic studies.

Scheme 2.59 meta ‐C–H deuteration of alcohols.
Source: Modified from Xu et al. [19].
2.2.7 Silane Derivatives
In 2016, Maiti and coworkers reported the Pd‐catalyzed meta ‐C–H olefination of synthetically versatile benzyl silanes using a nitrile‐based template ( Scheme 2.60) [53]. Sequential olefinations through performing selective mono‐olefination and bis‐olefination were also demonstrated for synthesizing valuable 2,5‐ or 3,5‐hetero divinylbenzene derivatives. Notably, the templated could be easily installed, and meta ‐olefinated toluene, benzaldehyde, and benzyl alcohols could be afforded while removing the silyl group with the template.
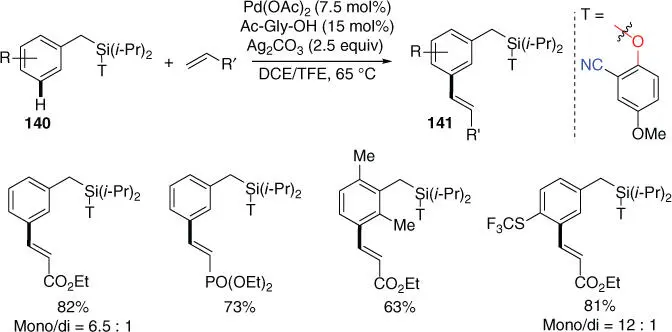
Scheme 2.60 meta ‐C–H olefination of benzyl silanes.
Source: Modified from Patra et al. [53].
Subsequently in 2017, Maiti and coworkers also developed a pyrimidine‐based template assisted meta ‐C–H cyanation of benzyl silanes using copper(I) cyanide as the cyanating agent ( Scheme 2.61a) [41]. The meta ‐cyano products are synthetically useful building blocks for preparing complex natural products as well as many drug molecules, and direct meta ‐C–H cyanation of benzyl silane and converting the silyl group to hydroxy group could be applied to the preparation of antidepressant drug citalopram ( Scheme 2.61b). It is worth mentioning that meta ‐C–H allylation was also feasible with this template for benzyl silanes, although only limited examples were disclosed.
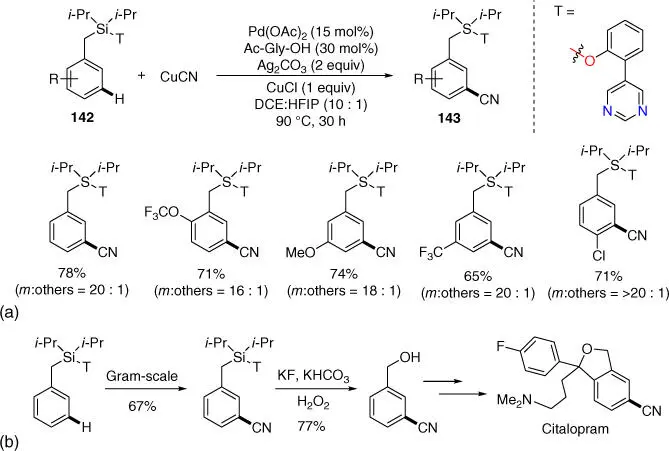
Scheme 2.61(a) meta ‐C–H cyanation of benzyl silanes. (b) Application of meta ‐C–H cyanation of benzyl silanes.
Source: (a) Modified from Bag et al. [41].
2.2.8 Phosphonate Derivatives
In 2016, Bera, Maiti, and coworker achieved the first Pd(II)‐catalyzed meta ‐C–H olefination of phosphonates at room temperature using the 2‐cyanophenol template ( Scheme 2.62) [54]. At elevated temperature, the reaction could be extended to meta ‐C–H hydroxylation or acetoxylation by varying the acetoxylating agent PhI(OCOR) 2. Notably, sequential di‐ meta ‐olefination would afford tri‐alkenylated arenes that could be used in organic electronics as well as optoelectronics.
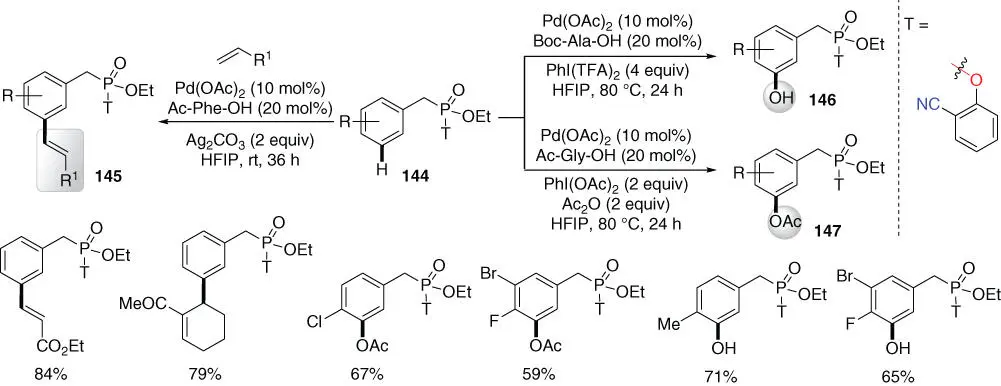
Scheme 2.62 meta ‐C–H functionalizations of phosphonates.
Source: Modified from Bera et al. [54].
Later in 2017, Maiti and coworkers achieved the meta ‐C–H alkylation of benzylphosphonates by using a pyrimidine‐based template using allyl alcohols, leading to the formation of β‐aryl aldehydes and ketones ( Scheme 2.63) [39]. Notably, the generality of this meta ‐alkylation had been demonstrated in phenethylsulfonyl ester as well as phenethyl carbonyl scaffolds as discussed earlier. Moreover, meta ‐C–H cyanation of benzylphosphonates was also achieved by using the same pyrimidine‐based template, albeit only two examples were disclosed [41].
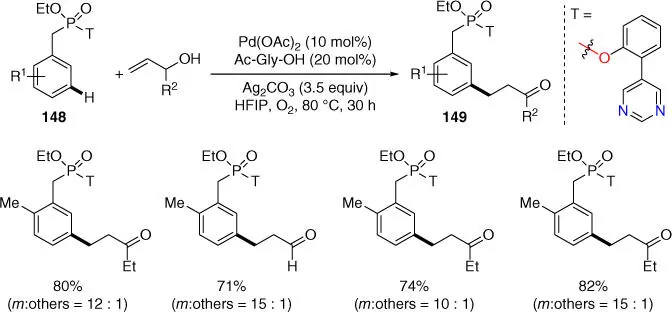
Scheme 2.63 meta ‐C–H alkylation of phosphonates.
Source: Modified from Bag et al. [39].
Phosphonates are useful synthons in the synthetic chemistry, since they could be transformed readily to the versatile alkenyl product by well‐established Horner–Wadsworth–Emmons reactions. And due to the importance of deuterium‐labeled compounds for pharmaceutical industry and reaction mechanistic studies, efficient direct deuteration methods would be very valuable. Recently, Yu, Dai, and coworkers developed palladium‐catalyzed meta ‐selective C–H deuteration of benzylphosphonates by using a pyridine‐based directing template ( Scheme 2.64) [19]. Notably, this method could be generally used for other substrates such as benzylsulfonates and benzyl alcohols via the practical ester linkage.
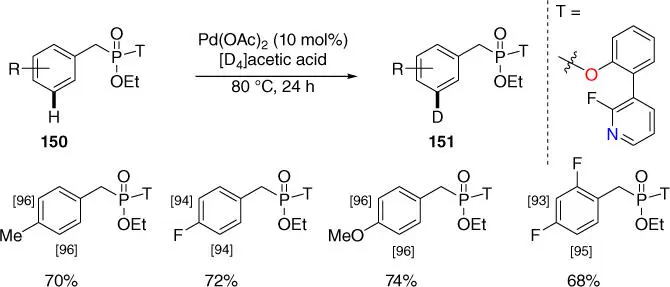
Scheme 2.64 meta ‐C–H deuteration of phosphonates.
Source: Modified from Xu et al. [19].
2.3 Mechanistic Considerations
The mechanisms of the aforementioned template assisted meta‐ C–H activation reactions are still not exactly very clear at present. However, detailed mechanistic investigations through computational and experimental mechanistic studies have been performed by such as the groups of Houk, Yu, and Wu, and the Maiti group to gain some hints of the reaction mechanism.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Remote C-H Bond Functionalizations»
Представляем Вашему вниманию похожие книги на «Remote C-H Bond Functionalizations» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Remote C-H Bond Functionalizations» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












