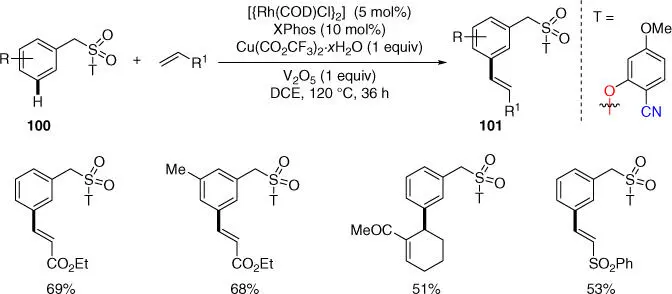
Scheme 2.39Rh‐catalyzed meta‐ C–H olefination of benzylsulfonyl esters.
Source: Modified from Bera et al. [22].
The development of efficient methods for the synthesis of organofluorine compounds is of great interest. Werz, Zanoni, Maiti, and coworkers achieved the first meta ‐C–H perfluoroalkenylation of benzylsulfonic acid derivatives using the pyrimidine‐based template with several commercially available perfluoroolefins ( Scheme 2.40) [24]. The substrate scope was broad and a high degree of compatibility with perfluoroolefins of different nature was also observed.
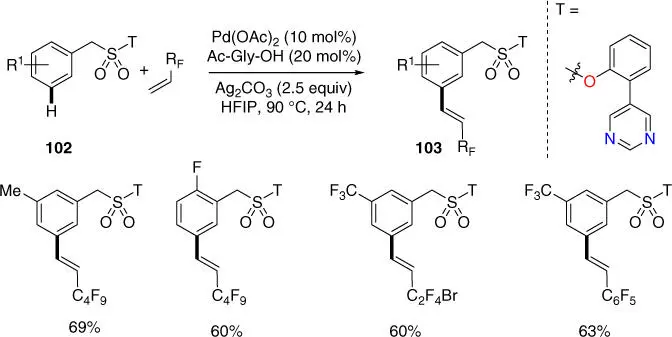
Scheme 2.40 meta‐ C–H perfluoroalkenylation of benzylsulfonyl esters.
Source: Modified from Brochetta et al. [24].
In 2019, Paton, Maiti, and coworkers developed the Palladium(II)‐catalyzed meta ‐selective C–H allylation of benzylsulfonyl esters using synthetically inert acyclic internal olefins as allylic surrogates ( Scheme 2.41) [27]. The pyrimidine‐based directing group proved to be the key factor to facilitate the olefin insertion by overcoming inertness of the typical unactivated internal olefins for meta ‐selective C–H activations. A broad scope of substrates with wide functional‐group tolerance was viable with this method to give good to excellent yields of products with exclusive E stereoselectivity.
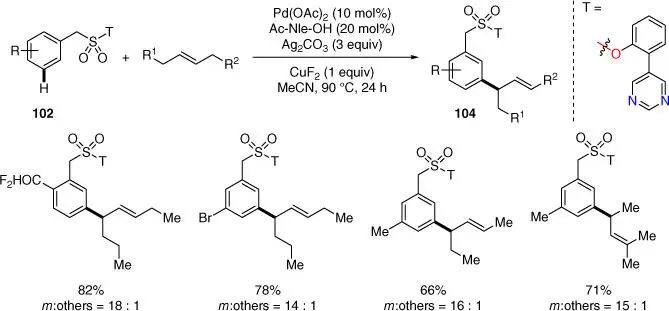
Scheme 2.41 meta‐ C–H allylation of benzylsulfonyl esters.
Source: Modified from Achar et al. [27].
Direct transformation of a C  H bond to a carbon–heteroatom bond is highly important since their prevalence in complex natural products, pharmaceuticals, and agrochemicals. In 2016, Sunoj, Maiti, and coworkers achieved meta‐ C–H oxygenation of benzylsulfonyl esters by using an oxygenating agent PhI(OOCR 3) 2( Scheme 2.42) [40]. Notably, the formation of hydroxylated or acetoxylated molecules was obtained under similar reaction conditions with the variation of R (R = H, F) on the common agent PhI(OOCR 3) 2. Importantly, the approach had also been applied to the synthesis of unsymmetrically substituted phenols.
H bond to a carbon–heteroatom bond is highly important since their prevalence in complex natural products, pharmaceuticals, and agrochemicals. In 2016, Sunoj, Maiti, and coworkers achieved meta‐ C–H oxygenation of benzylsulfonyl esters by using an oxygenating agent PhI(OOCR 3) 2( Scheme 2.42) [40]. Notably, the formation of hydroxylated or acetoxylated molecules was obtained under similar reaction conditions with the variation of R (R = H, F) on the common agent PhI(OOCR 3) 2. Importantly, the approach had also been applied to the synthesis of unsymmetrically substituted phenols.
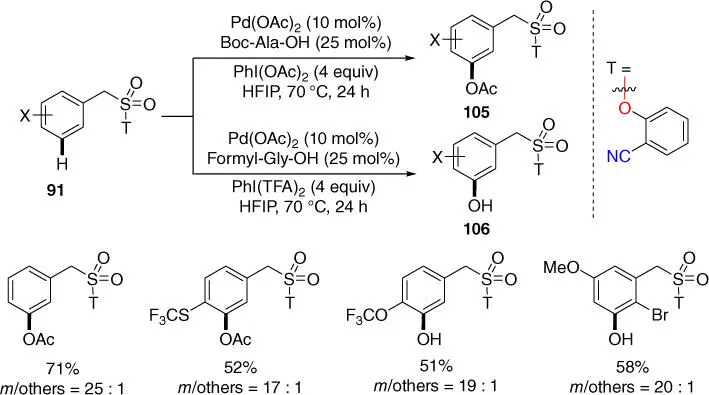
Scheme 2.42 meta‐ C–H oxygenation of benzylsulfonyl esters.
Source: Modified from Maji et al. [40].
In 2017, Maiti and coworkers disclosed a pyrimidine‐based template assisted meta ‐C–H cyanation of benzylsulfonyl and 2‐phenethylsulfonyl esters using stoichiometric amount of copper(I) cyanide as the cyanating agent ( Scheme 2.43) [41]. The meta ‐cyano products are useful building blocks for synthesis of complex natural products as well as many drug molecules, and the synthesis of pharmaceutically valuable precursors was demonstrated by using this novel protocol.
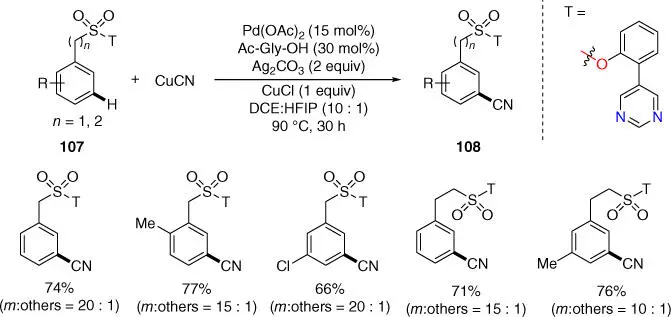
Scheme 2.43 meta‐ C–H cyanation of benzylsulfonyl and 2‐phenethylsulfonyl esters.
Source: Modified from Bag et al. [41].
Deuterium‐labeled compounds are important for pharmaceutical industry and kinetic studies of reaction mechanisms. In 2019, Werz, Maiti, and coworkers reported the Pd‐catalyzed meta‐ C–H deuteration of benzylsulfonyl esters derivatives using readily available deuterium source such as deuterated acetic acid, assisted by readily removable pyrimidine‐based template ( Scheme 2.44) [26]. It was demonstrated that the template morphology was crucial for effectivity and selectivity of this remote C  H bond activation. Meanwhile, Yu, Dai, and coworkers also achieved an isolated example of meta‐ C–H deuteration of benzylsulfonyl esters using pyridine‐based template.
H bond activation. Meanwhile, Yu, Dai, and coworkers also achieved an isolated example of meta‐ C–H deuteration of benzylsulfonyl esters using pyridine‐based template.
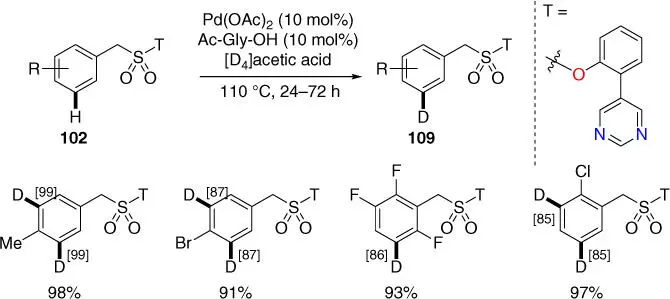
Scheme 2.44 meta‐ C–H deuteration of benzylsulfonyl esters.
Source: Modified from Bag et al. [26].
In 2017, the group of Maiti achieved an unprecedented meta ‐silylation and ‐germanylation of benzylsulfonyl esters by employing a nitrile‐based phenolic directing template using readily available hexamethyldisilane as the silylating agent ( Scheme 2.45) [42]. Notably, meta ‐silylation could be extended for 2‐phenylethanesulfonic acids and 3‐phenylpropane‐1‐sulfonic acid. Several examples of photosynthetic elaborations were attempted to demonstrate the synthetic utility, such as a formal synthesis of TAC101, a potential drug for the treatment of lung cancer.
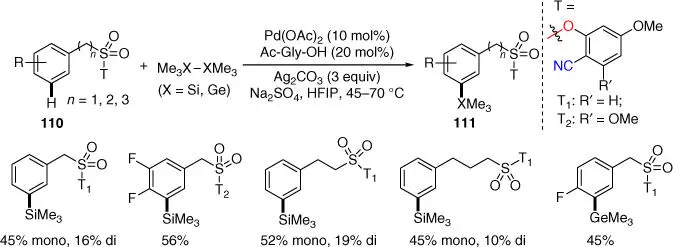
Scheme 2.45 meta‐ C–H silylation and germanylation.
Source: Modified from Modak et al. [42].
In 2013, Yu and coworkers achieved an unprecedented meta ‐C–H functionalization of electron‐rich phenol derivatives using the 2,2′‐azanediyldibenzonitrile directing template ( Scheme 2.46), leading to a synthetically useful method for meta ‐functionalizing α‐phenoxycarboxylic acids, the core structure of a fibrate class of drug molecules [43]. Remarkably, the selective C–H functionalization at the meta ‐positions of electron‐rich phenol derivatives was especially useful since it is orthogonal to previous electrophilic substitution of phenols in terms of regioselectivity. Notably, meta‐ C–H arylation of this class of phenol derivatives was also possible under the same meta ‐cross‐coupling conditions of hydrocinnamic acids derivatives with arylboronic esters, although only a limited number of examples were demonstrated.
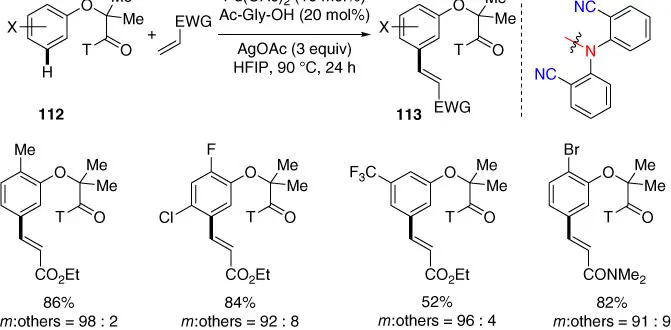
Scheme 2.46 meta‐ C–H olefination of phenol derivatives.
Later in 2017, Zhou, Xu, and coworkers developed a nitrile‐based organosilicon template assisted meta‐ C–H olefination of phenol derivatives in good yields with high meta‐ selectivities under similar reaction conditions ( Scheme 2.47) [44]. Importantly, the organosilicon linkage could be easily cleaved under mild conditions, and the directing template could be recovered under acidic conditions by using p ‐toluene sulfonic acid.
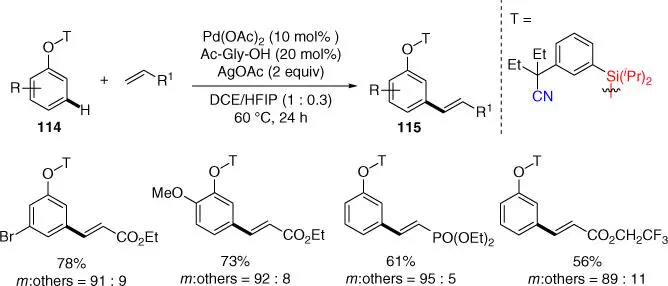
Scheme 2.47Organosilicon template assisted meta‐ C–H olefination of phenol derivatives.
Читать дальше




 H bond to a carbon–heteroatom bond is highly important since their prevalence in complex natural products, pharmaceuticals, and agrochemicals. In 2016, Sunoj, Maiti, and coworkers achieved meta‐ C–H oxygenation of benzylsulfonyl esters by using an oxygenating agent PhI(OOCR 3) 2( Scheme 2.42) [40]. Notably, the formation of hydroxylated or acetoxylated molecules was obtained under similar reaction conditions with the variation of R (R = H, F) on the common agent PhI(OOCR 3) 2. Importantly, the approach had also been applied to the synthesis of unsymmetrically substituted phenols.
H bond to a carbon–heteroatom bond is highly important since their prevalence in complex natural products, pharmaceuticals, and agrochemicals. In 2016, Sunoj, Maiti, and coworkers achieved meta‐ C–H oxygenation of benzylsulfonyl esters by using an oxygenating agent PhI(OOCR 3) 2( Scheme 2.42) [40]. Notably, the formation of hydroxylated or acetoxylated molecules was obtained under similar reaction conditions with the variation of R (R = H, F) on the common agent PhI(OOCR 3) 2. Importantly, the approach had also been applied to the synthesis of unsymmetrically substituted phenols.
















