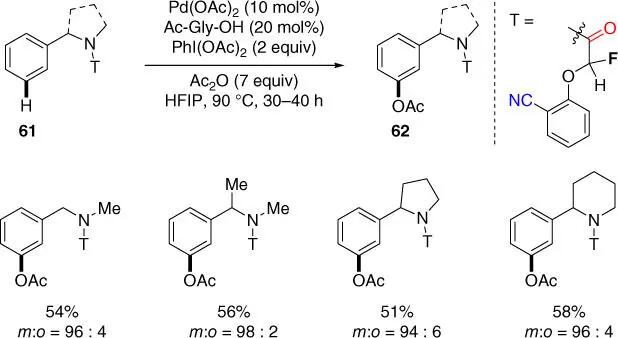
Scheme 2.24 meta‐ C–H acetoxylation of benzylamine derivatives.
Source: Modified from Tang et al. [31].
Besides meta ‐C–H acetoxylation of benzylamines, meta ‐C–H olefination was also reported by Xu, Xu, Jin, and coworkers through a quaternary ammonium salt assembly of the template for tertiary benzylamines [33]. Remarkably, this method was demonstrated to be applicable to several distal arene‐tethered tertiary amine derivatives, although only a few examples of benzylamine derivatives were tested ( Scheme 2.25).
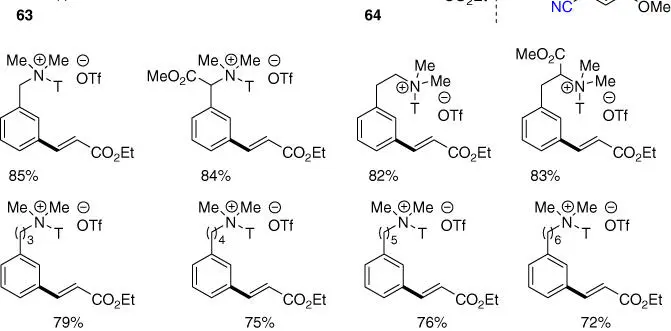
Scheme 2.25 meta‐ C–H olefination of tertiary benzylamines and distal arene‐tethered tertiary amine derivatives.
2.2.3.3 Phenylethylamine Derivatives
In 2015, the group of Li achieved the first meta ‐C–H olefination of N ‐methyl‐phenylethylamine derivatives using the 2‐cyanobenzoyl group as the CF ( Scheme 2.26) [34], followed by the isolated examples of meta ‐C–H olefination of tertiary phenylethylamine derivatives mentioned in Section 2.2.3.2[33]. In presence of Pd(OAc) 2and Ac‐Gly‐OH under nitrogen atmosphere, the reaction proceeded smoothly with a broad scope of substrate, and the directing template is structurally very simple and commercially available. Moreover, to increase the potential of application of direct C–H transformations in organic synthesis, using 2‐cyanobenzoyl group as the common original directing functionality to access regiodivergent C–H activation was also demonstrated in a sequential remote‐selective C–H olefination of 2‐fluorophenylethylamine ( Scheme 2.27). Notably, the first remote ‐selective C–H olefination occurs with the secondary amide 67leaving the proximal aromatic ortho ‐C  H bond intact in an ortho ‐selective manner and proceeds with an imidamide directing group that was formed through cyclization of the 2‐cyanobenzoyl motif. The desired meta‐ directing template was reconstructed with methylation by using LiHMDS as the base to afford substrate 69after hydrogenation. Finally, the second C–H olefination of 69led to the production of tetra‐substituted phenylethylamide 70in a meta ‐selective manner under standard reaction conditions, enabling the building of molecular complexity in a concise manner.
H bond intact in an ortho ‐selective manner and proceeds with an imidamide directing group that was formed through cyclization of the 2‐cyanobenzoyl motif. The desired meta‐ directing template was reconstructed with methylation by using LiHMDS as the base to afford substrate 69after hydrogenation. Finally, the second C–H olefination of 69led to the production of tetra‐substituted phenylethylamide 70in a meta ‐selective manner under standard reaction conditions, enabling the building of molecular complexity in a concise manner.
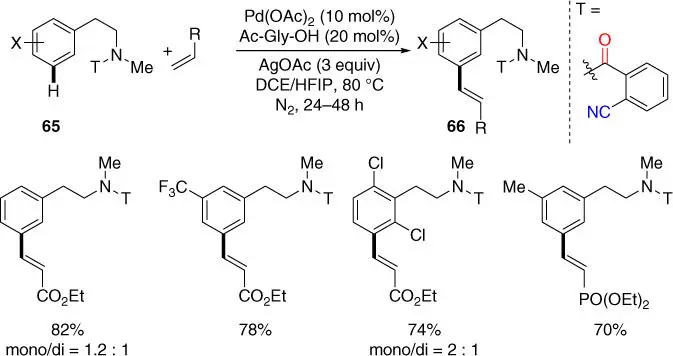
Scheme 2.26 meta‐ C–H olefination of phenylethylamine derivatives.
Source: Modified from Li et al. [34].
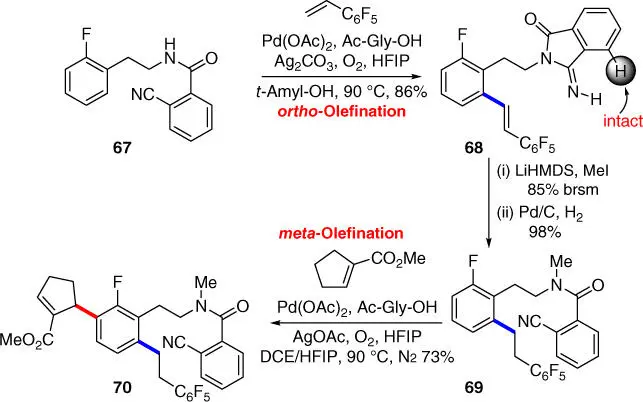
Scheme 2.27Sequential remote‐selective regiodivergent C–H olefination of 2‐fluorophenylethylamine.
2.2.3.4 N ‐Heterocyclic Arene Derivatives
Due to the prevalence of N ‐heterocycles in biologically important molecules, selective activation of a C  H bond of N ‐heterocyclic arenes is a conceptually intriguing and synthetically important challenge. For example, to selective activation of C
H bond of N ‐heterocyclic arenes is a conceptually intriguing and synthetically important challenge. For example, to selective activation of C  H bonds at C7 of bicyclic tetrahydroquinolines, a novel template will be required since a highly strained intermediate with a tricyclic cyclophane structure will be encountered. In 2014, Yu and coworkers found the template conformation was a critical factor in controlling remote meta ‐ or ortho ‐selectivity, and when a fluorine substituent was introduced into the auxiliary, the conformation that favored the meta ‐selectivity would be dominant in the presence of the MPAA ligand Ac‐Gly‐OH ( Scheme 2.28a) [31]. Intriguingly, when the substituents in the auxiliary scaffold were switched to methyl groups, high ortho ‐C–H activation at C7 of bicyclic tetrahydroquinolines was observed. With this new directing template by taking advantage of conformation control, remote meta ‐C–H olefination of tetrahydroquinolines was achieved in good yield and with high levels of site selectivity ( Scheme 2.28b).
H bonds at C7 of bicyclic tetrahydroquinolines, a novel template will be required since a highly strained intermediate with a tricyclic cyclophane structure will be encountered. In 2014, Yu and coworkers found the template conformation was a critical factor in controlling remote meta ‐ or ortho ‐selectivity, and when a fluorine substituent was introduced into the auxiliary, the conformation that favored the meta ‐selectivity would be dominant in the presence of the MPAA ligand Ac‐Gly‐OH ( Scheme 2.28a) [31]. Intriguingly, when the substituents in the auxiliary scaffold were switched to methyl groups, high ortho ‐C–H activation at C7 of bicyclic tetrahydroquinolines was observed. With this new directing template by taking advantage of conformation control, remote meta ‐C–H olefination of tetrahydroquinolines was achieved in good yield and with high levels of site selectivity ( Scheme 2.28b).
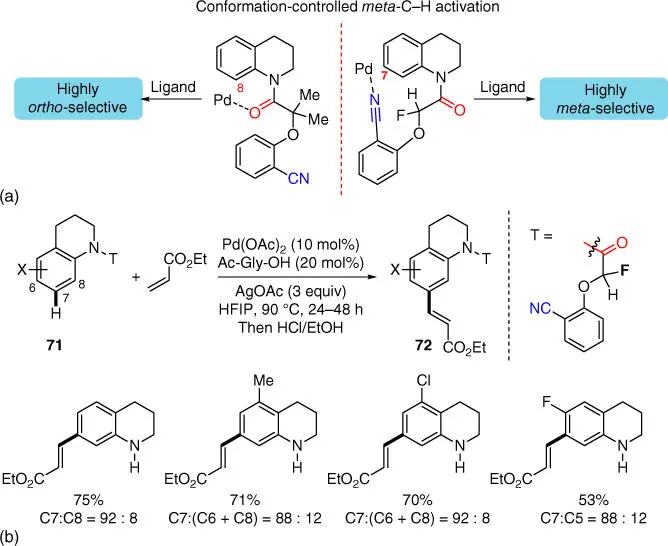
Scheme 2.28(a) Conformation promoted meta ‐selective activation. (b) meta‐ C–H olefination of tetrahydroquinoline derivatives.
Source: (a) Modified from Tang et al. [31].
Subsequently, Movassaghi, Yu, and coworkers developed a novel nitrile‐based sulfonamide directing template for indolines, since previous directing template for tetrahydroquinolines was not viable presumably due to that the aryl group of indolines is more electron rich and the new skeleton requires a different template to accommodate ( Scheme 2.29a) [35]. The new electronically withdrawing sulfonamide linkage is crucial for the meta‐ selective C–H functionalization of electron‐rich indolines that are otherwise highly reactive toward electrophilic palladation at the electron‐rich C5‐positions. With this new template and the established reaction conditions, a range of synthetically useful and advanced indoline analogues were efficiently olefinated at the C6 position, meta to the nitrogen atom. Moreover, the sulfonamide template could be removed at room temperature with magnesium turnings in methanol to afford meta‐ alkylated indoline derivatives through simultaneous reduction of the newly installed olefins ( Scheme 2.29b).
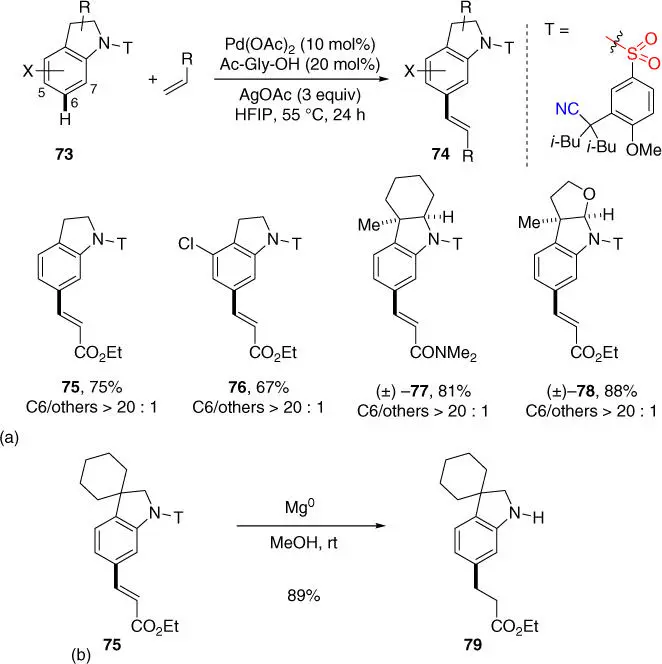
Scheme 2.29(a) meta‐ C–H olefination of indoline derivatives. (b) Removal of directing template.
Source: (a) Modified from Yang et al. [35].
Based on the success of meta‐ C–H olefination of electron‐rich indolines, the meta‐ C–H arylation of indolines under previously reported meta‐ C–H cross‐coupling reaction conditions for hydrocinnamic acid derivatives was also achieved, demonstrating the versatility of this new template for diverse meta‐ C–H functionalizations of indolines ( Scheme 2.30) [35]. Several indoline substrates reacted with arylboronic acid pinacol esters to afford meta ‐arylated indoline derivatives in synthetically useful yields.
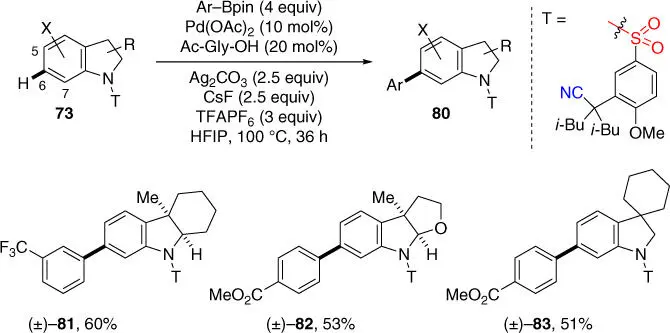
Scheme 2.30 meta‐ C–H arylation of indoline derivatives.
Source: Modified from Yang et al. [35].
Читать дальше



 H bond intact in an ortho ‐selective manner and proceeds with an imidamide directing group that was formed through cyclization of the 2‐cyanobenzoyl motif. The desired meta‐ directing template was reconstructed with methylation by using LiHMDS as the base to afford substrate 69after hydrogenation. Finally, the second C–H olefination of 69led to the production of tetra‐substituted phenylethylamide 70in a meta ‐selective manner under standard reaction conditions, enabling the building of molecular complexity in a concise manner.
H bond intact in an ortho ‐selective manner and proceeds with an imidamide directing group that was formed through cyclization of the 2‐cyanobenzoyl motif. The desired meta‐ directing template was reconstructed with methylation by using LiHMDS as the base to afford substrate 69after hydrogenation. Finally, the second C–H olefination of 69led to the production of tetra‐substituted phenylethylamide 70in a meta ‐selective manner under standard reaction conditions, enabling the building of molecular complexity in a concise manner.















