Remote C-H Bond Functionalizations
Здесь есть возможность читать онлайн «Remote C-H Bond Functionalizations» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Remote C-H Bond Functionalizations
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Remote C-H Bond Functionalizations: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Remote C-H Bond Functionalizations»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Remote C—H Bond Functionalizations
Remote C—H Bond Functionalizations
Remote C-H Bond Functionalizations — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Remote C-H Bond Functionalizations», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
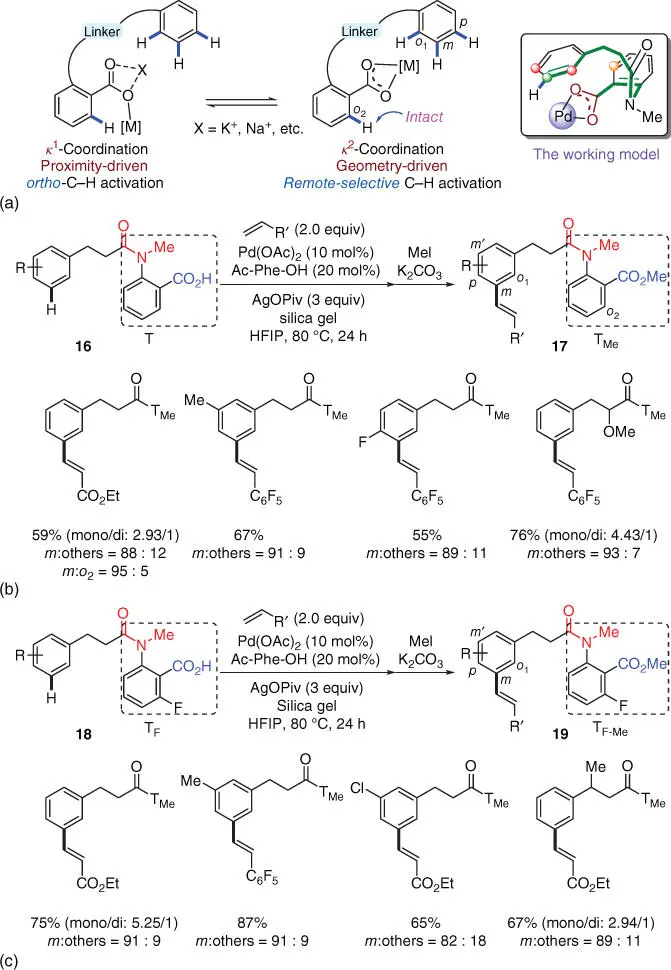
Scheme 2.6(a) Proposed remote‐selective C‐H activation via κ2 coordination of the carboxyl. (b) Remote‐selective meta ‐C–H olefination of hydrocinnamic acids. (c) Improved site‐selectivity and reactivity with a modified carboxyl‐containing template.
Source: (a) Modified from Li et al. [15].
Besides Pd‐catalysts, Rh‐catalyst could also be used for olefination of hydrocinnamic acids. In 2017, Lu, Sun, Yu, and coworkers reported the first example of Rh(III)‐catalyzed, directing template assisted remote meta ‐C–H olefination of hydrocinnamic acids via a postulated 12‐membered macrocyclic intermediate ( Scheme 2.7a) [16]. The directing template bearing a single nitrile group was slightly different from Yu's seminal template. Moreover, molecular oxygen could be used as the terminal oxidant for this reaction. Subsequently, meta ‐C–H alkenylation of hydrocinnamic acids was also realized using alkynes, which are significantly less reactive than the polarized and reactive acrylate ( Scheme 2.7b) [17]. Notably, transition metal‐catalyzed meta ‐alkenylation using alkynes has not been successful with Pd catalysts in previous reports on the directing template strategy.
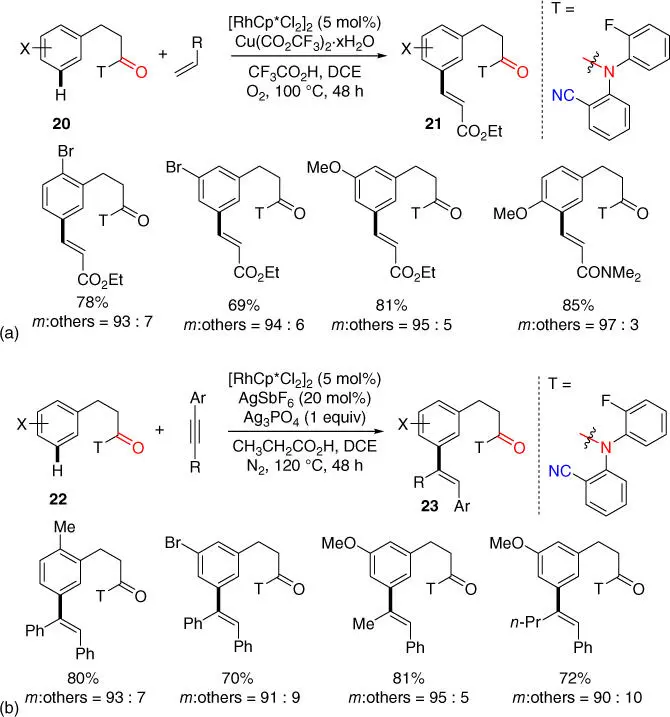
Scheme 2.7(a) Rh(III)‐catalyzed directing template assisted remote meta ‐C–H olefination of hydrocinnamic acids. (b) Rh(III)‐catalyzed meta ‐C–H alkenylation of hydrocinnamic acids using alkynes.
Source: (a) Modified from Xu et al. [16]; (b) Modified from Xu et al. [17].
In addition to meta‐ C–H olefination, meta‐ C–H arylation of hydrocinnamic acids was made possible using the directing template strategy. In 2013, Yu and coworkers reported the first example of Pd‐catalyzed cross‐coupling of meta‐ C  H bonds with arylboronic esters to afford meta ‐arylated hydrocinnamic acids derivatives ( Scheme 2.8) [18]. A more electron‐rich nitrile‐based directing template with methoxy substitutions was found to better assist this reaction. Moreover, the privileged solvent HFIP and the MPAA ligand (Ac‐Gly‐OH) previously used for meta‐ C–H olefination of hydrocinnamic acid was still crucial for the reaction. Notably, tetrabutylammonium (TBA) salt tetrabutylammonium hexafluorophosphate (TBAPF 6), which might prevent undesired agglomeration of Pd(0) species to give unreactive palladium black, dramatically improved the reaction yield. It is worth mentioning that biaryl compounds are import structural motifs that are frequently found in numerous pharmaceuticals and agrochemicals.
H bonds with arylboronic esters to afford meta ‐arylated hydrocinnamic acids derivatives ( Scheme 2.8) [18]. A more electron‐rich nitrile‐based directing template with methoxy substitutions was found to better assist this reaction. Moreover, the privileged solvent HFIP and the MPAA ligand (Ac‐Gly‐OH) previously used for meta‐ C–H olefination of hydrocinnamic acid was still crucial for the reaction. Notably, tetrabutylammonium (TBA) salt tetrabutylammonium hexafluorophosphate (TBAPF 6), which might prevent undesired agglomeration of Pd(0) species to give unreactive palladium black, dramatically improved the reaction yield. It is worth mentioning that biaryl compounds are import structural motifs that are frequently found in numerous pharmaceuticals and agrochemicals.
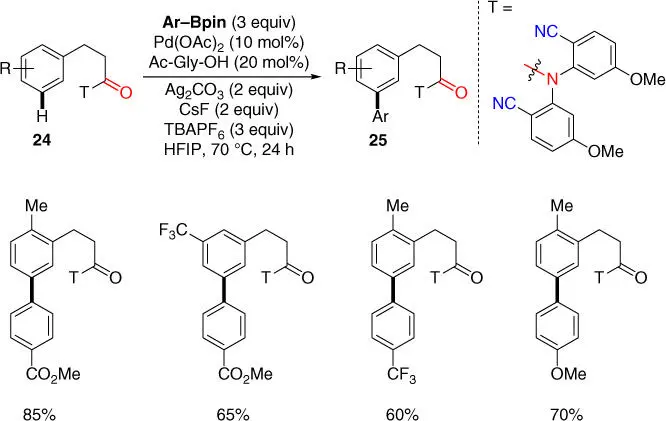
Scheme 2.8 meta‐ C–H arylation of hydrocinnamic acids with arylboronic esters.
Source: Modified from Wan et al. [18].
Despite the success of meta‐ C–H arylation with arylboronic esters, meta‐ C–H arylation using low‐cost aryl iodides proved to be challenging with the nitrile‐based templates. Most recently, Li and coworkers achieved the first example of meta‐ C–H arylation of hydrocinnamic acid derivatives with aryl iodides assisted by the carboxyl‐based template ( Scheme 2.9) [15]. Notably, the site‐selectivity is generally excellent with moderate to good yields. Moreover, aryl halides bearing an ortho ‐electron‐withdrawing group ( o ‐EWG) such as a methyl ester group were found to be most suitable with the reaction, which is possibly because that the combined electronic as well as weak coordinating effect of the o ‐EWG could facilitate oxidative addition with these aryl iodides.
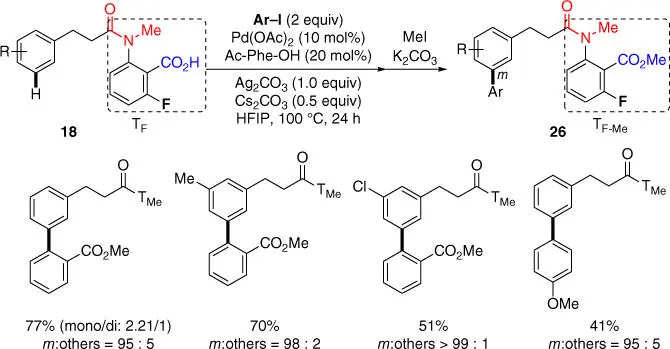
Scheme 2.9 meta‐ C–H arylation of hydrocinnamic acids with aryl iodides.
Source: Modified from Li et al. [15].
Finally, C–H deuteration and alkylation of hydrocinnamic acid derivatives were also possible using N ‐based heteroarene‐containing templates, but only isolated examples were disclosed [19]. In short, the notable meta‐ C–H activation hydrocinnamic acid derivatives includes olefination and arylation with Pd(II) or Rh(III) catalysts, using nitrile‐based or carboxyl‐based templates.
2.2.2.2 Phenylacetic Acid Derivatives
The generality of the template strategy in accommodating potential macrocyclopalladation processes with smaller ring sizes was also investigated with phenylacetic acid derivatives. Notably, the Fujiwara–Moritani‐type olefination is often used as the effective model reaction to test feasibility of a new substrate design of meta‐ C–H activation relation. In 2014, the Maiti group introduced a new category of nitrile‐based phenolic directing template, which is now available from Sigma Aldrich as the Maiti–Bera–Modak (MBM) auxiliary, for meta ‐C–H olefination of phenylacetic acid derivatives via an ester linkage ( Scheme 2.10) [20]. In this new class of substrates, a smaller 12‐membered cyclic transition state was proposed for the palladation step than hydrocinnamic acid derivatives. With this easily synthesized and removable 2‐hydroxybenzonitrile template, a broad range of phenylacetates were olefinated in a highly mono‐selective as well as meta ‐selective fashion. Moreover, this protocol was also applied to drug molecules such as ibuprofen in a moderate yield and selectivity, products of which are difficult to access using conventional methods of diversification.
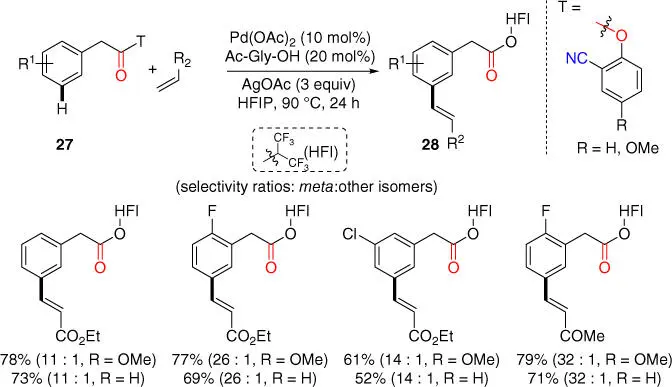
Scheme 2.10 meta‐ C–H olefination of phenylacetate.
Source: Modified from Bera et al. [20].
Simultaneously, Yu and coworker disclosed a protocol of the commercially available dibenzonitrile directing template assisted meta‐ C–H olefination of phenylacetic acid derivatives via an amide linkage [21]. Notably, N ‐formyl‐protected glycine (Formyl‐Gly‐OH) was identified as the new ligand for this new class of substrate. Unlike previous protocols, weak base KH 2PO 4was required to use as the crucial additive. This reaction further demonstrates the good versatility of the template approach in accommodating potentially necessary macrocyclopalladation processes with different ring size for different classes of substrates ( Scheme 2.11) [21].
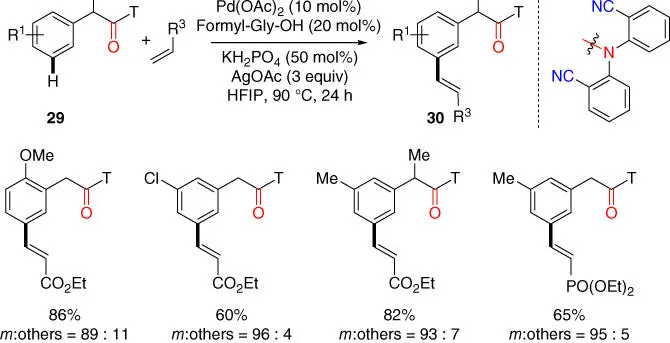
Scheme 2.11 meta‐ C–H olefination of phenylacetic acid derivatives. Source: Modified from Deng et al. [21].
In 2017, the Maiti group developed the first rhodium‐catalyzed meta ‐C–H olefination of phenylacetic acid frameworks with the assistance of 2‐hydroxy‐4‐methoxy benzonitrile template ( Scheme 2.12) [22]. The XPhos (2,4′,6′‐diisopropyl‐1,1′‐biphenyl‐2‐yldicyclohexylphosphine) was identified as the crucial ligand for this reaction. Substituents of phenylacetic acid esters at all positions of the arene ring were found to be tolerated. Synthetic application of this protocol was also expanded to the diversification of ketoprofen, a drug molecule containing a secondary α‐methyl substituent.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Remote C-H Bond Functionalizations»
Представляем Вашему вниманию похожие книги на «Remote C-H Bond Functionalizations» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Remote C-H Bond Functionalizations» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












