Remote C-H Bond Functionalizations
Здесь есть возможность читать онлайн «Remote C-H Bond Functionalizations» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Remote C-H Bond Functionalizations
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Remote C-H Bond Functionalizations: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Remote C-H Bond Functionalizations»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Remote C—H Bond Functionalizations
Remote C—H Bond Functionalizations
Remote C-H Bond Functionalizations — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Remote C-H Bond Functionalizations», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
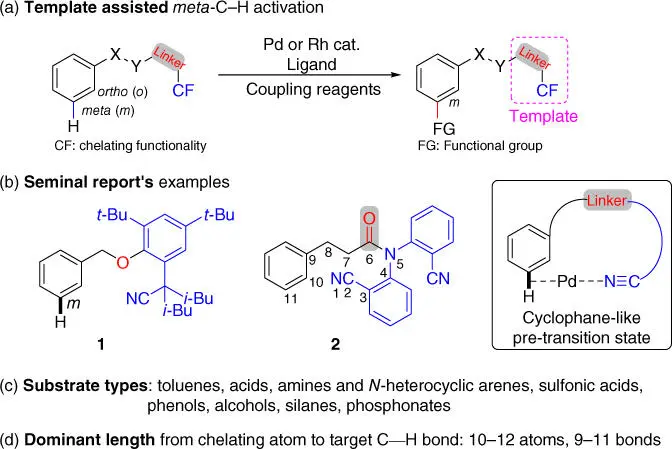
Scheme 2.1Directing template assisted meta ‐C  H bond functionalization. Related reviews:
H bond functionalization. Related reviews:
(a) Li et al. [5], Yang [6], Chattopadhyay and Bisht [7], Dey et al. [8], Ghosh and De Sarkar [9], and Dey et al. [10]; Source: (b) Modified from Leow et al. [11].
Inspired by this pioneering work, a series of directing template assisted remote meta ‐C–H activation reactions have been realized for a list of substrates including acids, amines, sulfonic acids, and so on ( Scheme 2.1c). Notably, one of the key features of these reactions is the target C  H bond is usually 10–12 atoms away from the chelating atom of the template ( Scheme 2.1b,d), although longer length was also possible. To date, three categories of CFs have been engineered including two nitrogen‐based CN‐containing ( Scheme 2.2a) or heteroarene‐containing ( Scheme 2.2b) CFs and one oxygen‐based CO 2H‐containing CF ( Scheme 2.2c). It should be noted that besides these three CFs that covalently attached to the substrate, two catalytic bifunctional templates that reversibly coordinate to the substrate were also reported recently and they are not classified in these categories [12,13]. Another key feature of these reactions is that hexafluoroisopropanol (HFIP), which could also be used as an additive, appears to be the privileged solvent. Finally, N ‐acetyl glycine (Ac‐Gly‐OH), a mono‐ N ‐protected amino acid (MPAA), is often the ligand of choice for many of these reactions, although other MPAA ligands could also be utilized in some cases.
H bond is usually 10–12 atoms away from the chelating atom of the template ( Scheme 2.1b,d), although longer length was also possible. To date, three categories of CFs have been engineered including two nitrogen‐based CN‐containing ( Scheme 2.2a) or heteroarene‐containing ( Scheme 2.2b) CFs and one oxygen‐based CO 2H‐containing CF ( Scheme 2.2c). It should be noted that besides these three CFs that covalently attached to the substrate, two catalytic bifunctional templates that reversibly coordinate to the substrate were also reported recently and they are not classified in these categories [12,13]. Another key feature of these reactions is that hexafluoroisopropanol (HFIP), which could also be used as an additive, appears to be the privileged solvent. Finally, N ‐acetyl glycine (Ac‐Gly‐OH), a mono‐ N ‐protected amino acid (MPAA), is often the ligand of choice for many of these reactions, although other MPAA ligands could also be utilized in some cases.
Herein, we summarize important achievements that were disclosed until October 2019 in the field of directing template assisted meta ‐C–H functionalization of arenes with Pd or Rh catalysts since 2012. Different aspects of this type of methodology will be covered while discussing the works that are categorized by the substrate type. Important mechanistic studies on this methodology will also be included. It is hoped that the reader will learn the key points, especially structural features, for rationally designing a feasible template for new substrates as well as developing new types of meta ‐C–H transformation after reading this chapter.
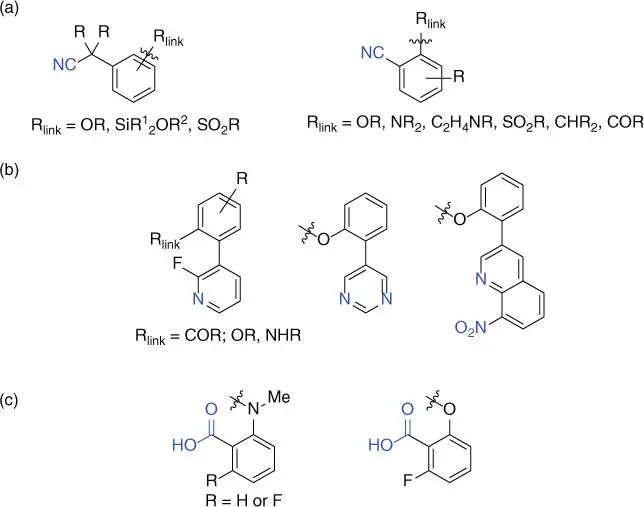
Scheme 2.2Three categories of chelating functionality (CF). (a) N‐BasedCN‐containing CF; (b) N‐basedheteroarene‐containing CF; (c) O‐basedCO 2H‐containing CF.
2.2 Template‐Assisted meta‐C–H Functionalization
2.2.1 Toluene Derivatives
In 2012, Yu and coworkers devised the first effective U‐shaped nitrile‐based directing template that was covalently linked to the toluene derivatives via a removable benzyl ether linkage ( Scheme 2.3) [11]. Notably, the directing ability of the template was improved by installing two isobutyl templates at the α‐position adjacent to the linearly chelating nitrile template due to the Thorpe–Ingold effect. This directing template efficiently enabled the meta‐ C–H olefination of a broad range of toluene derivatives using Pd(OPiv) 2as the catalyst and AgOPiv as the oxidant. It is worth mentioning that such remote C–H activation that possibly demanded a cyclophane‐like 11‐membered palladacycle was first ever disclosed. Remarkably, the intrinsic electronic and steric biases of the substrates were successfully overridden. Finally, the directing template was readily cleaved through hydrogenolysis with a Pd/C catalyst.
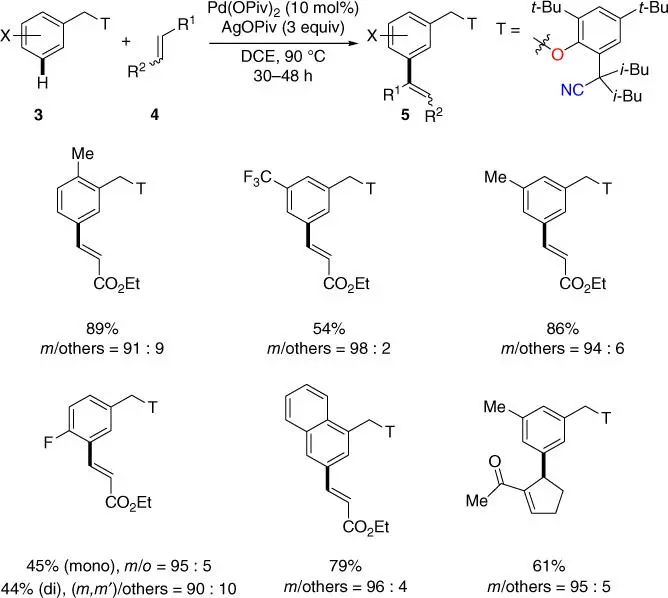
Scheme 2.3 meta‐ C–H activation of toluene derivatives.
Source: Modified from Leow et al. [11].
2.2.2 Acid Derivatives
2.2.2.1 Hydrocinnamic Acid Derivatives
In Yu's seminal report, meta‐ C–H olefination of hydrocinnamic acid derivatives was also achieved using an easily synthesized and recyclable 2,2′‐azanediyldibenzonitrile directing template, which is now available from Sigma–Aldrich as the Yu–Li auxiliary [11]. This template was attached to several hydrocinnamic acids via a readily cleavable amide linkage ( Scheme 2.4). It is worth mentioning that hydrocinnamic acids are core motifs of many drug molecules such as Baclofen. Notably, it was discovered that the MPAA ligand Ac‐Gly‐OH from the simplest amino acid significantly improved the yield of the reaction and improved the selectivity as well with the optimal HFIP solvent that was crucial for the full conversion of the substrate. It was found in subsequent reports that this set of novel reaction conditions was also highly effective for many of the directing templated assisted meta‐ C–H transformations. In this transformation, not only the intrinsic electronic biases of the substrate were overridden ( 9, 10), but also challenging steric hindrance was overcome by the template ( 11). Intriguingly, biaryl acid substrate that has the same length between the chelating nitrile group and the target meta‐ C  H bond as hydrocinnamic acid could also undergo meta‐ selective C–H olefination of the remote aryl ring ( 12). Finally, the directing template could be easily removed by hydrolysis under basic conditions at room temperature, leading to the meta‐ olefinated hydrocinnamic acids and the recycled directing template.
H bond as hydrocinnamic acid could also undergo meta‐ selective C–H olefination of the remote aryl ring ( 12). Finally, the directing template could be easily removed by hydrolysis under basic conditions at room temperature, leading to the meta‐ olefinated hydrocinnamic acids and the recycled directing template.
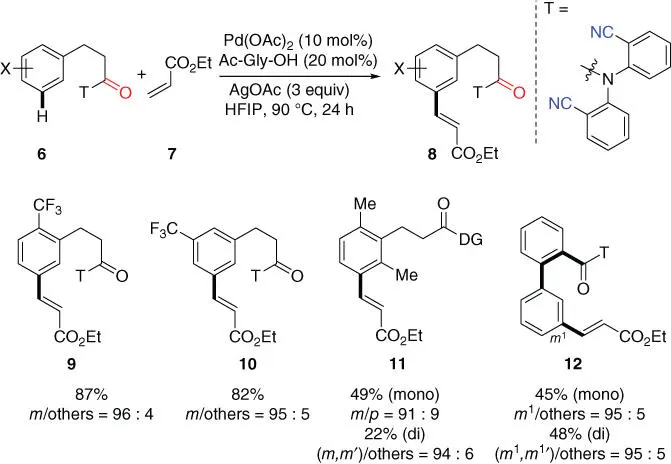
Scheme 2.4 meta‐ C–H olefination of hydrocinnamic acid derivatives.
In 2016, Maiti and coworkers disclosed a 2‐hydroxybenzonitrile template for mono meta ‐selective olefination of hydrocinnamic acids via an ester linkage ( Scheme 2.5a) [14]. Due to the less statistical availability of the coordinating nitrile group compared with the original 2,2′‐azanediyldibenzonitrile directing template, high mono‐/di‐selectivity could be obtained with this new template at lower reaction temperature using 1,2‐dichloroethane (DCE) as the major solvent and HFIP as the minor solvent. Notably, hetero‐di‐olefination product could be afforded with a second meta ‐C–H olefination under similar reaction conditions using HFIP as the sole solvent ( Scheme 2.5b).
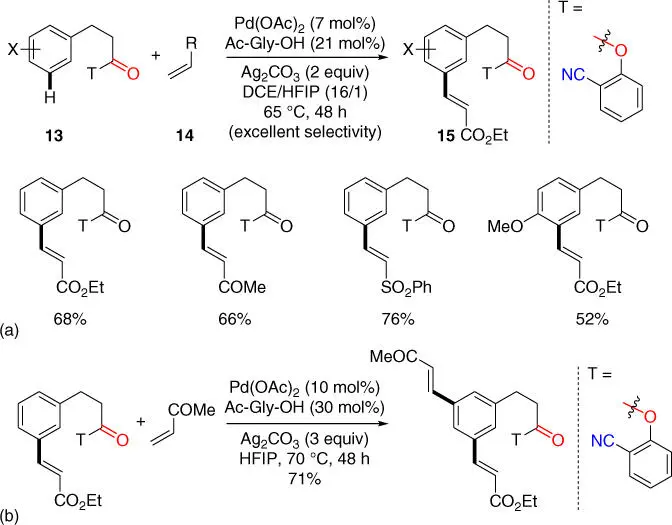
Scheme 2.5(a) 2‐hydroxybenzonitrile template assisted mono meta ‐selective olefination of hydrocinnamic acids; (b) meta ‐selective hetero‐di‐olefination of hydrocinnamic acids.
Source: (a) Modified from Modak et al. [14].
Most recently, Li and coworkers developed the first example of carboxy group assisted, remote meta ‐selective C(sp 2)–H activation with a Pd II‐catalyst via potential κ 2coordination of the carboxyl, suppressing the ortho ‐C–H activation via the κ 1coordination ( Scheme 2.6a) [15]. Unlike the previous nitrogen‐based CN‐containing or heteroarene‐containing templates, this is the first oxygen‐based carboxyl‐containing template, whose coordination geometry could be considered as a pseudo‐linear coordination along the aryl–CO 2M bond similar to the nitrile‐coordination geometry. Notably, hydrocinnamic acids could be meta ‐olefinated in a remote selective fashion, leaving the C  H bond ortho to the carboxy group on the same aryl intact, although carboxy group is well‐known to be a good ortho ‐directing group ( Scheme 2.6b). Moreover, tuning the electronic and steric properties of the carboxy group by switching the hydrogen atom ortho to the carboxy group with a fluorine atom, the yield as well as the site selectivity could be improved to some extent ( Scheme 2.6c). The possible presence of κ 2coordination in assisting remote‐selective C–H activation may inspire the exploration of novel site‐selectivity of the carboxyl assisted C–H activation reactions.
H bond ortho to the carboxy group on the same aryl intact, although carboxy group is well‐known to be a good ortho ‐directing group ( Scheme 2.6b). Moreover, tuning the electronic and steric properties of the carboxy group by switching the hydrogen atom ortho to the carboxy group with a fluorine atom, the yield as well as the site selectivity could be improved to some extent ( Scheme 2.6c). The possible presence of κ 2coordination in assisting remote‐selective C–H activation may inspire the exploration of novel site‐selectivity of the carboxyl assisted C–H activation reactions.
Интервал:
Закладка:
Похожие книги на «Remote C-H Bond Functionalizations»
Представляем Вашему вниманию похожие книги на «Remote C-H Bond Functionalizations» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Remote C-H Bond Functionalizations» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












