Remote C-H Bond Functionalizations
Здесь есть возможность читать онлайн «Remote C-H Bond Functionalizations» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Remote C-H Bond Functionalizations
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Remote C-H Bond Functionalizations: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Remote C-H Bond Functionalizations»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Remote C—H Bond Functionalizations
Remote C—H Bond Functionalizations
Remote C-H Bond Functionalizations — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Remote C-H Bond Functionalizations», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Since the meta ‐hydroxylated indolines are biologically important, meta ‐C–H acetoxylation of indolines using the well‐established oxidation conditions was also investigated ( Scheme 2.31) [35]. It was found that although the meta ‐acetoxylated indolines were obtained as the major products, the meta ‐selectivity was much lower than olefination and para ‐acetoxylated indolines (∼10%) were also formed probably due to the electrophilic palladation at the electron‐rich C5 position under the reaction conditions. It should be mentioned that the nonsubstituted indoline substrate was not compatible with these oxidation conditions, since the indoline was readily oxidized to give a mixture of unidentified compounds instead. Further investigation of this reaction to find better reaction conditions is required.
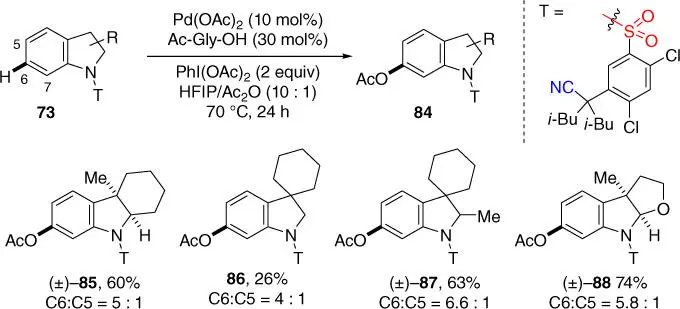
Scheme 2.31 meta‐ C–H acetoxylation of indoline derivatives.
Source: Modified from Yang et al. [35].
In 2018, Yu and coworkers developed a novel simple ortho ‐sulfonyl benzonitrile template to achieve remote ortho or meta ‐C–H olefination of six different classes of N ‐heterocycles, including indoline, indole, and tetrahydroquinoline ( Scheme 2.32) [37]. It was demonstrated that the site‐selectivity could be predicted based on distance and geometry, and template‐directed meta ‐C–H activation was possible through macrocyclopalladation processes with smaller ring size (10‐membered organometallic ring for indoline and indole).
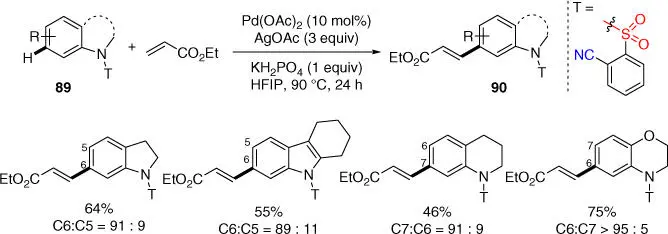
Scheme 2.32 meta‐ C–H olefination of N ‐heterocycles.
Source: Modified from Dutta et al. [36].
Stoichiometric installation of the template is not step‐economic, or it would not be attached to the substrate while there is no appropriate functional group to form the covalent linkage. Thus, the design of a catalytic template that binds the substrate via a reversible coordination instead of a covalent linkage is very desirable. In 2017, Yu and coworkers reported a remarkable breakthrough for meta‐ C–H olefination of 3‐phenylpyridines by using a catalytic pyridine‐based bifunctional template ( Scheme 2.33) [12]. In this reaction, the novel template coordinated two metal centers, one of which one reversibly anchored substrates near the catalyst. The other metal cleaved the meta ‐C–H selectively. Notably, unlike previous reaction conditions, the addition of the new copper acetate additive was crucial, since both the yield and the meta ‐selectivity would decrease greatly in the absence of copper acetate.
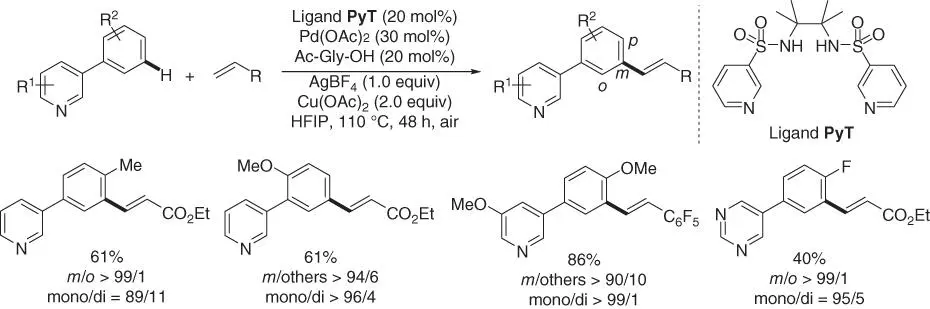
Scheme 2.33 meta‐ C–H olefination of 3‐phenylpyridines.
Source: Modified from Zhang et al. [12].
Subsequently, Maiti and coworkers reported a novel nitrile‐based bifunctional template for meta‐ C–H olefination of 3‐phenylpyridines with Pd(acac) 2as the catalyst under similar reaction conditions ( Scheme 2.34) [13]. Notably, this nitrile‐based bis‐amide template was easily prepared, which was beneficial for its application in the synthesis of complex molecular structures.
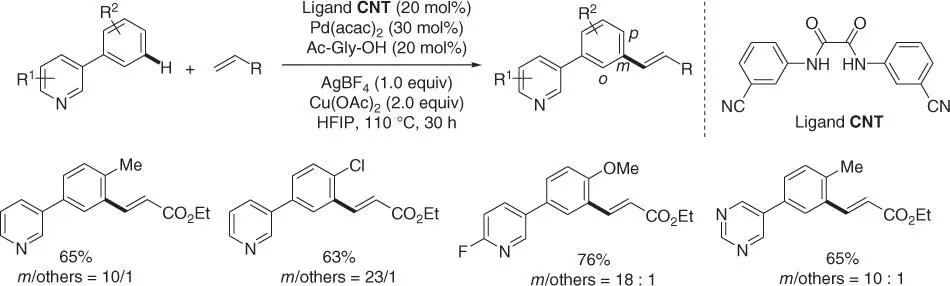
Scheme 2.34 meta‐ C–H olefination of 3‐phenylpyridines using nitrile‐based bifunctional template.
Source: Modified from Achar et al. [13].
2.2.4 Sulfonic Acid Derivatives
In 2015, Maiti and coworkers developed the first meta‐ C–H activation of benzylsulfonic acid derivatives using aforementioned commercially available the 2‐hydroxybenzonitrile directing template with high and controllable mono‐selectivity ( Scheme 2.35a) [38]. Thus, meta‐ selective homo‐diolefination and sequential hetero‐diolefination of benzylsulfonyl ester derivatives were made possibly with this protocol, providing a novel method for the synthesis of hetero‐dialkenylated products that are difficult to access using conventional methods. Notably, the directing template could be converted to an alkenyl group using modified Julia olefination conditions ( Scheme 2.35b).
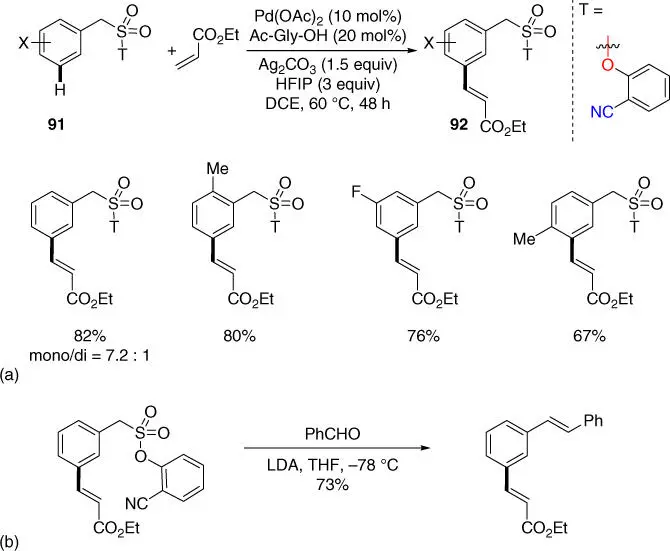
Scheme 2.35(a) meta‐ C–H olefination of benzylsulfonic acid derivatives. (b) Elaboration of product via olefination.
Source: (a) Modified from Bera et al. [38].
Subsequently, Maiti and coworkers also achieved the meta‐ C–H olefination of 2‐phenethylsulfonic acid derivatives using the similar approach as the benzylsulfonic acid using the N ‐formyl glycine (For‐Gly‐OH) ligand ( Scheme 2.36a) [14]. Notably, sequential meta ‐selective hetero‐diolefination was also feasible with these substrates, and the deprotection and recovery of the directing template were successfully realized under basic conditions ( Scheme 2.36b).
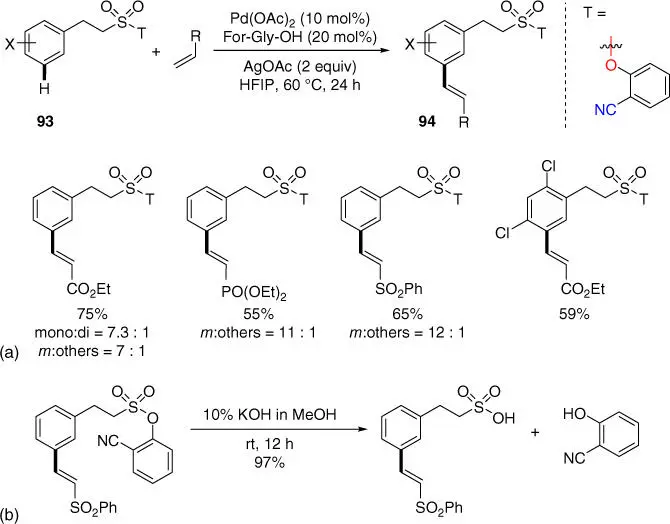
Scheme 2.36(a) meta‐ C–H olefination of 2‐phenethylsulfonic acid derivatives. (b) Removal of directing template.
Source: (a) Modified from Modak et al. [14].
In 2017, Maiti and coworkers disclosed the first pyrimidine‐based template, which is effective for meta ‐C–H olefination of benzylsulfonyl and 2‐phenethylsulfonyl esters, leading to an unconventional formation of α,β‐unsaturated aldehydes with benzyl‐protected allyl alcohols ( Scheme 2.37) [39]. This novel pyrimidine‐based template was also utilized for the production of β‐aryl aldehydes and ketones, using allyl alcohols through meta ‐C–H alkylation of benzylsulfonyl and 2‐phenethylsulfonyl esters.
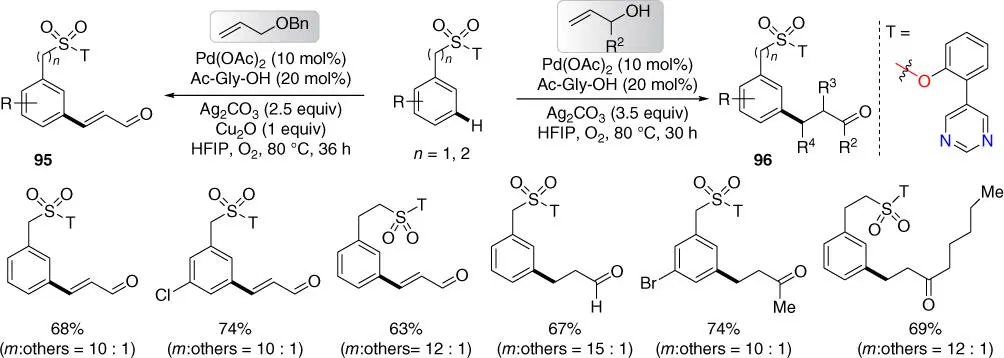
Scheme 2.37 meta‐ C–H olefination and alkylation of benzylsulfonyl and 2‐phenethylsulfonyl esters.
Source: Modified from Bag et al. [39].
In the same year, the Maiti group introduced the novel 8‐nitroquinoline‐based directing template for meta‐ C–H functionalization of benzylsulfonyl esters via strong σ‐coordination, ensuring the potential formation of a stable palladacycle ( Scheme 2.38) [36]. The protocol enabled both meta‐ C–H olefination and acetoxylation, which could be scaled up and diversified to synthetically important compounds in late stage.
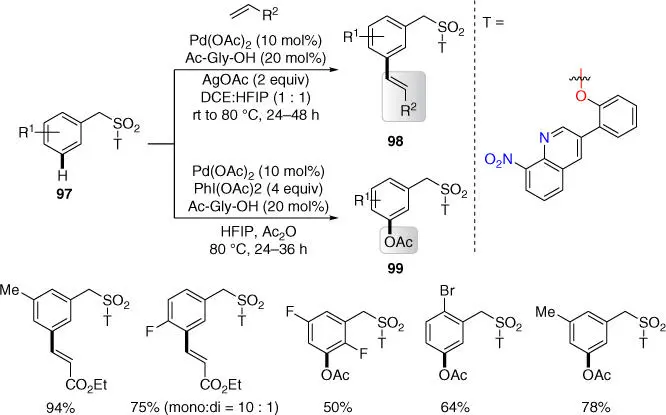
Scheme 2.38 meta‐ C–H olefination and acetoxylation of benzylsulfonyl esters.
Source: Modified from Dutta et al. [36].
In 2017, Maiti and coworkers also developed the XPhos‐supported Rhodium catalysis of meta‐ C–H olefination of benzylsulfonic acids with the assistance of 2‐hydroxy‐4‐methoxy benzonitrile template ( Scheme 2.39) [22]. Complete mono ‐selectivity was observed for this reaction, and a range of olefins and substituents at all positions of the aryl ring were found to be tolerated.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Remote C-H Bond Functionalizations»
Представляем Вашему вниманию похожие книги на «Remote C-H Bond Functionalizations» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Remote C-H Bond Functionalizations» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












