Starting from diglycidyl ethers of 2‐methoxyhydroquinone and vanillyl alcohol through an epoxy ring opening with ammonia, it is possible to obtain two primary amines, which could be applied as epoxy resin cross‐linking agents. Because of β‐hydroxyl groups present in the molecules of diamines, they exhibit the autocatalytic effect on the epoxy–amine reactions. These new β‐hydroxylamines can be used for cross‐linking of diglycidyl ether of methoxyhydroquinone and diglycidyl ether of vanillyl alcohol giving fully bio‐based epoxy systems with good thermomechanical properties and high thermal stability.
Cardanol derivatives are classified as the phenolic curing agents that are cross‐linked with epoxy groups via the phenolic hydroxyl group. Novolac resins (Nov‐I and Nov‐II), containing an amount of unreacted cardanol of 35 and 20 wt%, respectively, are synthesized by the condensation reaction of cardanol and paraformaldehyde using oxalic acid as a catalyst ( Figure 1.63) [149].
The cardanol‐based novolacs might be used as curing agents of commercial (the diglycidyl ether of bisphenol A) epoxy resin in the presence of 2‐ethyl‐4‐methyl‐imidazole as a catalyst. The higher cross‐linking density is observed with higher amounts of epoxy resin. Moreover, the resin cured with Nov‐II is characterized by higher T gand better mechanical properties than Nov‐I‐resin. On the other hand, because of the higher molecular weight and lower unreacted cardanol content, the similar thermal degradation properties are observed for both tested materials.
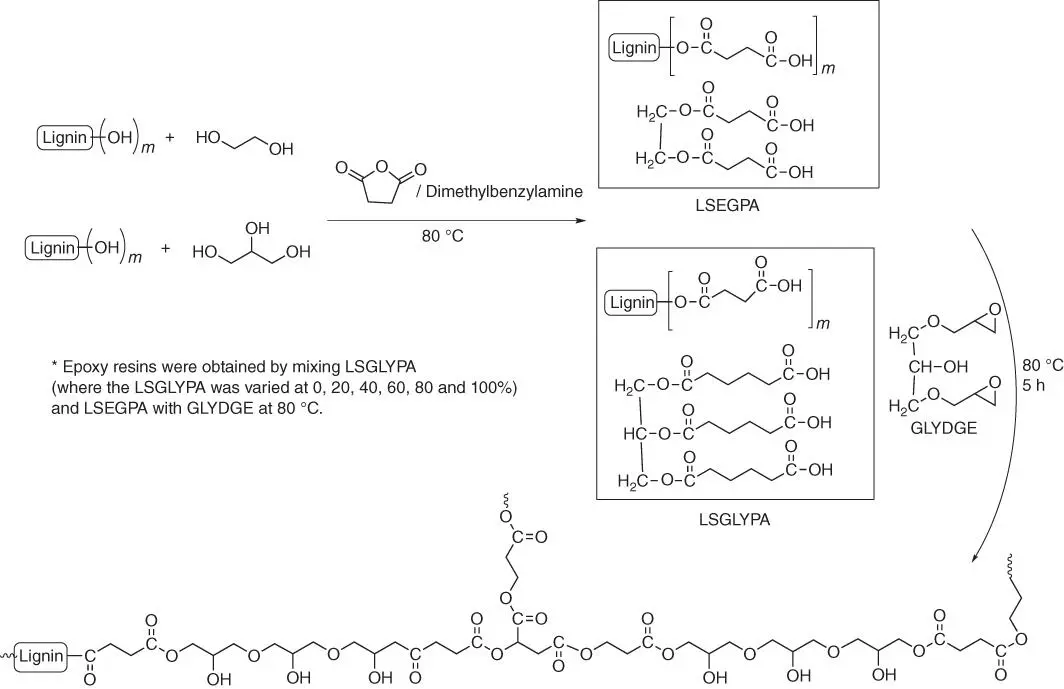
Figure 1.60 Scheme of preparation cross‐linked epoxy resin.
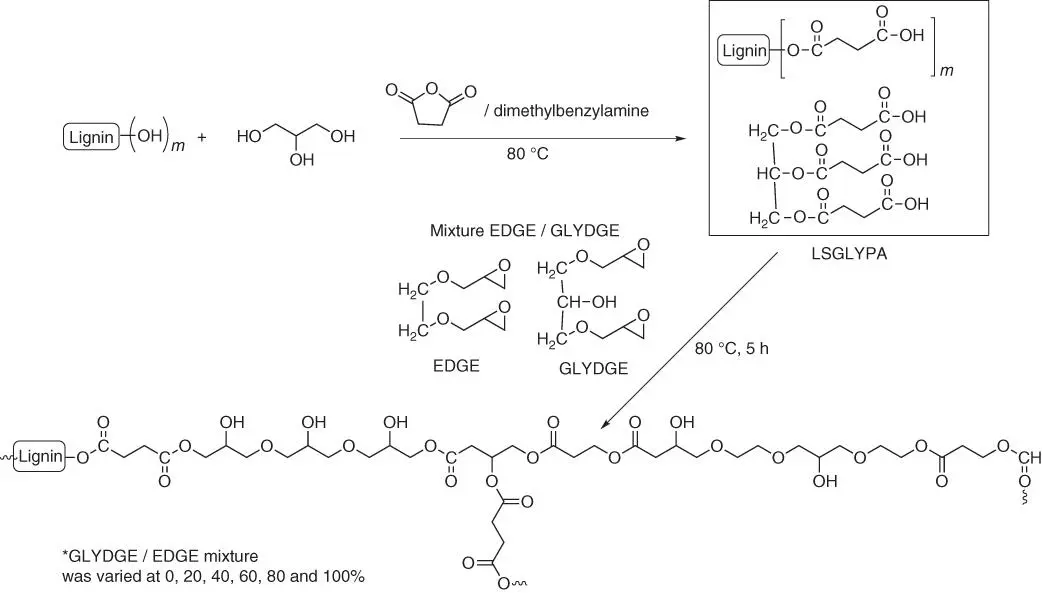
Figure 1.61 Scheme of epoxy resin by cross‐linking LSGLYPA with a mixture of EGDGE/GLYDGE.
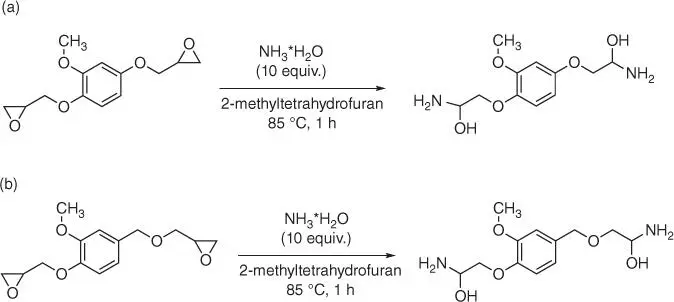
Figure 1.62 Synthesis of dihydroxyaminopropane of 2‐methoxyhydroquinone (a) and (b) vanillyl alcohol.
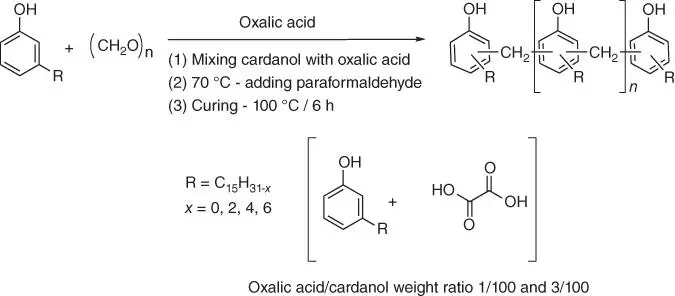
Figure 1.63 Synthesis of the cardanol‐based novolacs.
The interesting cross‐linking agent is possible to obtain as the diamine derivative of isosorbide ( Figure 1.64) and it could be used for curing of diglycidyl ether of isosorbide giving the completely bio‐based epoxy system [150].
The diamine derivative of isosorbide is obtained using microwave assistant thiol‐ene coupling reaction in the aqueous media and with the water‐soluble initiator (NH 4) 2S 2O 8, as the alternative to AIBN. The cured isosorbide‐based resin has good shape fixity, good shape recovery, and satisfied thermal stability. This fully bio‐based resin shows great potential to be used as a candidate for shape memory material. Another synthetic method for obtaining the diamine derivative of isosorbide is the reaction of cyanoethylation of isosorbide, followed by the hydrogenation of di‐cyanoethylated product ( Figure 1.65) [151].
Terpene derivatives might also be applied as bio‐based curing agents for epoxy resins. For example, a novel terpene‐based curing agent prepared as an adduct of myrcene, monoterpene containing three double bonds including conjugated diene, and maleic anhydride (MMY) is used to cure bisphenol A‐based epoxy resin [152]. The obtained cured material is characterized by a tensile strength of 48.74 MPa and a storage modulus of 19.06 MPa, but a poor impact property of 8.55 kJ/m 2and a very low elongation at break (7.54%). Improvement of properties of cured bio‐based epoxy resins is performed by application of mixture (mixed by weight ratios of 100/0, 75/25, 50/50, 25/75, and 0/100, respectively) of two curing agents: MMY and castor oil‐modified adduct of myrcene and maleic anhydride (CMMY) ( Figure 1.66).
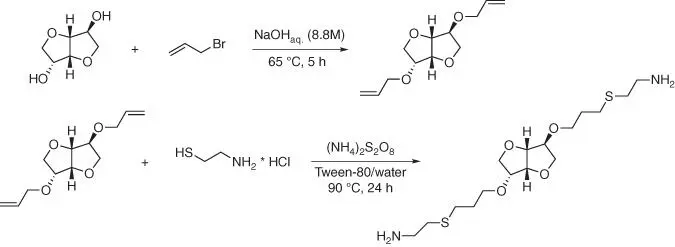
Figure 1.64 Synthesis of the isosorbide‐based cross‐linking agent.
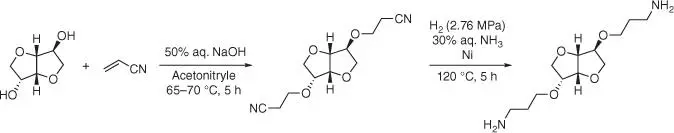
Figure 1.65 Synthesis of the isosorbide‐based cross‐linking agent via cyanoethylation step.
Table 1.7 Properties of epoxy resin networks cured with bio‐based curing agents.
| Cured samples |
T g(°C) |
E at T g+30°C(MPa) |
Tensile strength (MPa) |
Elongation at break (%) |
Impact strength (kJ/m 2) |
| MMY100 |
61.59 |
19.06 |
48.74 |
7.5 |
8.55 |
| MMY75/CMMY25 |
58.03 |
19.00 |
42.11 |
6.5 |
13.87 |
| MMY50/CMMY50 |
54.03 |
4.18 |
35.55 |
6.6 |
17.29 |
| MMY25/CMMY75 |
45.09 |
2.72 |
11.11 |
259.4 |
62.51 |
| CMMY100 |
15.14 |
1.11 |
0.43 |
565.8 |
Unbroken |
Based on the obtained results ( Table 1.7), with the increase of CMMY weight ratio, the tensile strength and T gof the cross‐linked resin decreases, but the elongation at break and the impact strength increase.
As can be seen from the examples presented above, it is possible to obtain not only epoxy resins from raw materials of natural origin but also cross‐linking agents for them. Interestingly, it is also possible to synthesize completely bio‐derived epoxy systems. Both the epoxy resins based on bisphenol A, as well as cross‐linked with curing agents on the basis of raw materials of natural origin, and the fully epoxy biosystems are characterized by very good final properties.
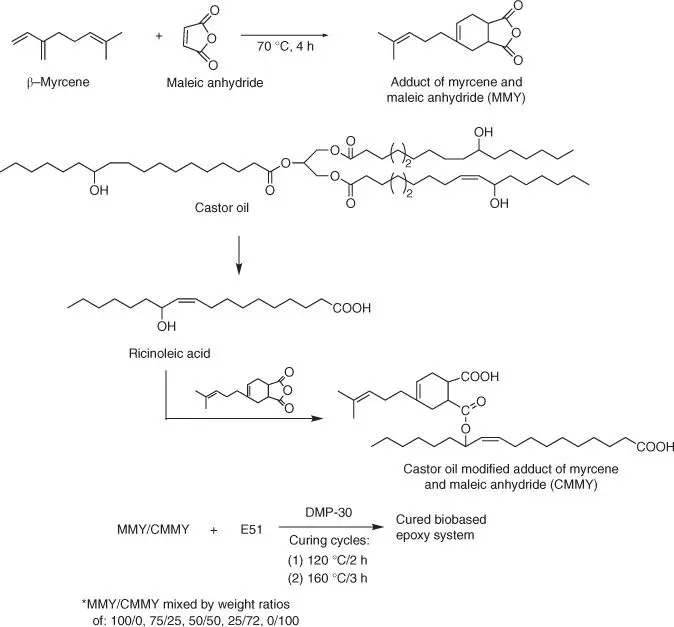
Figure 1.66 Synthesis of bio‐based epoxy curing agent derived from myrcene and castor oil.
1 1 Derksen, J.T.P., Cuperus, F.P., and Kolser, P. (1996). Prog. Org. Coat. 27 (1–4): 45–53.
2 2 Sharma, V. and Kundu, P.P. (2006). Prog. Polym. Sci. 31 (11): 983–1008.
3 3 Köckritz, A. and Martin, A. (2008). Eur. J. Lipid Sci. Technol. 110: 812–824.
4 4 Hilker, I., Bothe, D., Prüss, J., and Warnecke, H.‐J. (2001). Chem. Eng. Sci. 56: 427–432.
5 5 Chen, J., de Liedekerke Beaufort, M., Gyurik, L. et al. (2019). Green Chem. 21: 2436–2447.
6 6 Sienkiewicz, A.M. and Czub, P. (2016). Ind. Crops Prod. 83: 755–773.
7 7 Chua, S.Ch., Xu, X., and Guo, Z. (2012). Process Biochem. 47: 1439–1451.
8 8 Gan, L.H., Goh, S.H., and Ooi, K.S. (1992). J. Am. Oil Chem. Soc. 69 (4): 347–351.
9 9 Faria‐Machado, A.F., Altenhofen da Silva, M., Vieira, M.G.A., and Beppu, M.M. (2013). J. Appl. Polym. Sci. 127 (5): 3543–3549.
10 10 Walton, H.W. and Nevin, Ch.S. (1965). Reaction of half esters of an alpha, beta‐ethylenically unsaturated‐alpha, beta‐dicarboxylic acid with a vicinal epoxy compound and products thereof. US Patent 3, 190, 899, assigned to A.E. Staley Manufacturing Company, June 22, 1965.
Читать дальше



















