Modified lignin derivatives are also applied as curing agents for the epoxy resins. Commercially, various curing agents for epoxy resins are available. The commonly used petroleum‐based curing agents include amines, amides, hydroxyls, acid anhydrides, phenols, and polyphenols. However, recently, a great effort has been put on obtaining new bio‐based hardeners for epoxy systems. Generally, lignin‐based curing agents are prepared by two different methods: (i) the reaction of lignin with ozone in the presence of NaOH to give lignin with unsaturated carboxyl groups ( Figure 1.57) or (ii) throughout the reaction of modified lignin (partially depolymerized lignin or polyol solutions of alcoholysis lignin) with anhydrides or trimellitic anhydride chloride [144].
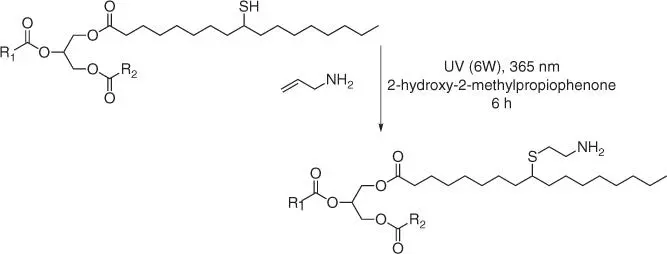
Figure 1.56 Synthesis of a polyamine cross‐linking agent via thiol‐ene reaction of mercaptanized soybean oil with allylamine.

Figure 1.57 Synthesis of carboxylic acid from lignin.
Another interesting approach is using aminated lignin (black powder, amine value: 180–200 mg KOH/g) as a cross‐linker of bisphenol A‐based epoxy resin (epoxy value: 0.48–0.54 mol/100 g) [145]. The obtained aminated lignin contains a large number of primary and secondary amine groups, which successfully cure the epoxy network ( Figure 1.58).
The application of aminated lignin has the positive effect at the initial degradation stage of the epoxy resin. Because lignin itself has a good thermal–mechanical performance, samples prepared with its higher content presented accordingly improved properties ( Table 1.6).
TGA and DMA tests reveal improved thermal–mechanical properties of the bio‐based epoxy resin. In comparison to the epoxy resin cross‐linked with the commercial curing agent based on modified isophorone diamine (W93 curing agent: amine value: 550–600 mg KOH/g), the recorded value of T 10, for the material cured with the hardener containing 20 wt% of aminated lignin, increased nearly by 50 °C, while the highest value of T 10was achieved for the epoxy system cured by 100% aminated lignin ( T 10= 266 °C for 100% of petrochemical‐based hardener W93, T 10= 315 °C and T 10= 332 °C for aminated lignin hardener, 80%W93 + 20%AL and 100%AL, respectively). Additionally, the mass loss before 300 °C of the epoxy resin cured by W93 is four times higher than the one recorded for the aminated lignin. Moreover, the obtained materials are characterized by improved values of the glass transition temperature and thermal deformation temperature. T gand T dof epoxy resin sample cured with the hardener containing 50 wt% of lignin increased by 14 °C compared with the one without lignin.

Figure 1.58 The curing of epoxy resin using aminated lignin as a curing agent.
Table 1.6 TGA, T g, and T dvalues of epoxy resin samples cured with different contents of lignin in the curing agent [145].
| Epoxy resin cured by different hardeners |
Total mass loss before 300 °C (%) |
T 10(°C) |
T 50(°C) |
T g(°C) |
T d(°C) |
| 100%W93 |
11.1 |
266 |
362 |
79 |
70 |
| 80%W93 + 20%AL |
8.7 |
315 |
370 |
86 |
74 |
| 70%W93 + 30%AL |
— |
— |
— |
89 |
76 |
| 60%W93 + 40%AL |
7.2 |
325 |
364 |
92 |
79 |
| 50%W93 + 50%AL |
— |
— |
— |
93 |
84 |
| 40%W93 + 60%AL |
8.4 |
317 |
371 |
— |
— |
| 20%W93 + 80%AL |
5.5 |
327 |
363 |
— |
— |
| 100%AL |
3.7 |
332 |
372 |
— |
— |
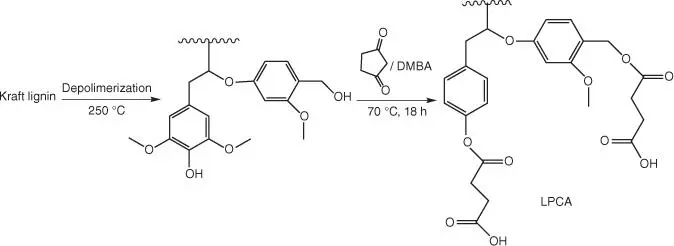
Figure 1.59 Preparation of partially depolymerized lignin (PDL) and lignin polycarboxylic acid LPCA) from Kraft lignin.
Another interesting utilization of lignin‐based compounds for the curing purposes of epoxy resin is application of partially depolymerized Kraft lignin [146]. In order to increase its solubility in organic solvents, lignin is subjected to the base‐catalyzed depolymerization in supercritical methanol. The resulting partially depolymerized lignin (PDL) is then converted to lignin‐based polycarboxylic acid (LPCA) by reacting with succinic anhydride ( Figure 1.59).
LPCA might be applied as a curing or co‐curing agent for epoxy resins. The curing of a commercial epoxy (DER353, epoxy value: 0.500–0.526 mol/100 g) using LPCA is conducted in the presence of 1 wt% of ethyl‐4‐methyl‐imidazole as a catalyst and at the similar temperature range to the commercial hexahydrophthalic anhydride (HHPA). The obtained cured material exhibits a moderate T gand comparable storage modulus to that cross‐linked with a commercial anhydride curing agent. Additionally, linear succinate monoester, used in the synthesis of LPCA, enhances the flexibility of the lignin molecules. Therefore, increasing the content of bio‐curing agent in the formulation tends to reduce the T gof cured resins. For composition of DER353/LPCA with equivalent ratio 1/0.6, 1/0.8, to 1/1, the T gof the cured resins decreases from 78.5 and 69.4 to 62.3 °C, while the storage moduli at room temperature is comparable (2.4–2.7 GPa). Based on the studies on the application of the solid LPCA together with other liquid curing agents, such as glycerol tris (succinate monoester) and commercial hexahydrophthalic anhydride to cure epoxies, it was observed that using a mixture of LPCA and a liquid curing agent not only adjusts the viscosity of the resin system but also significantly regulates the dynamic mechanical properties and thermal stability of the obtained epoxy materials.
Moreover, interesting two different types of novel cross‐linked epoxy resins from lignin and glycerol are reported [147]. The first one is obtained by mixing the product of sodium lignosulfonate (LS) and glycerol (LSGLYPA, where the content of LSGLYPA varies at 0, 20, 40, 60, 80, and 100%) with polyacid of sodium lignosulfonate and ethylene glycol (LSEGPA) and ethylene glycol diglycidyl ether (EGDGE) at 80 °C ( Figure 1.60).
The second one is by mixing LSGLYPA with a mixture of EGDGE/GLYDGE (the content of EGDGE/GLYDGE mixture was 0, 20, 40, 60, 80, and 100%) under similar reaction conditions ( Figure 1.61).
The glass transition temperature of the cross‐linked epoxy resins increases with increasing LSGLYPA and GLYDGE contents. The increase of T gfor the product of the first synthesis is due to the increase in cross‐linking density. In turn, for the second sample, an increase in the GLYDGE content increases T gof the cured epoxy resins (hydrogen bonding became the dominant factor).
Vanillin can be converted into the diamine derivatives ( Figure 1.62) [148].
Читать дальше
















