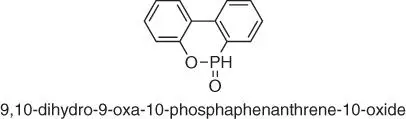
Figure 1.38 Chemical structure of DOPO.
Based on the performed studies ( Table 1.5), it was observed that the incorporation of PTCP into DGEBA epoxy resin accelerates the thermal degradation process and improves the char yield.
The same higher char yield is valuable toward improving the flame‐retardant properties of epoxy resins. The LOI value of EP/PTCP‐30% increases from 23.0% (neat epoxy material) to 30.5%. Moreover, compared with neat epoxy resin, the impact strength of EP/PTCP‐30% increases by 29%.
Cardanol is an interesting nonedible by‐product of CNSL industry. Because of the presence of phenolic hydroxyl group, olefinic linkages in the alkyl chain, and aromatic ring, it is a promising nonharmful renewable substituent to BPA. The presence of long aliphatic chain of cardanol in bio‐based epoxy resins results in lower T gvalues of obtained materials than those with DGEBA. At the same time, these materials are characterized by very interesting thermal stabilities. Moreover, the mechanical properties of cardanol‐based epoxies are lower than DGEBA epoxies. However, numerous, described in the literature, studies tested coating applications of epoxies obtained with the cardanol derivatives.
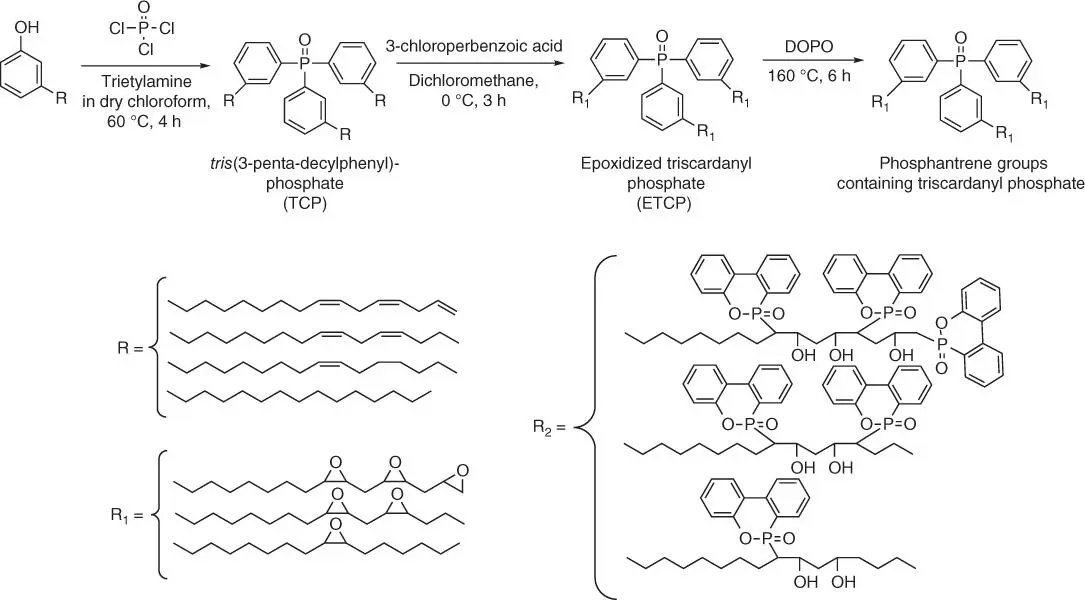
Figure 1.39 Synthesis of PTCP.
Table 1.5 Thermal and mechanical properties of PTCP, neat epoxy, and EP/PTCP samples.
| Sample |
Thermal properties |
Mechanical properties |
|
T 10%(°C) |
T max1(°C) |
T max2(°C) |
Char residue (%) |
LOI (%) |
Impact strength (kJ/m 2) |
Tensile modulus (GPa) |
Tensile strength (MPa) |
Elongation at break (%) |
| PTCP |
286 |
322 |
500 |
2.8 |
— |
— |
— |
— |
— |
| EP |
361 |
367 |
554 |
1.4 |
23.0 |
14.9 ± 1.1 |
1.56 ± 0.10 |
40.6 ± 2.5 |
2.2 ± 0.8 |
| EP/PTCP‐10% |
336 |
344 |
554 |
4.2 |
26.5 |
16.8 ± 1.0 |
1.35 ± 0.00 |
46.3 ± 3.4 |
5.5 ± 01 |
| EP/PTCP‐20% |
328 |
344 |
575 |
5.7 |
28.0 |
18.1 ± 1.4 |
1.46 ± 0.03 |
60.8 ± 4.4 |
7.7 ± 1.8 |
| EP/PTCP‐30% |
311 |
335 |
577 |
8.3 |
30.5 |
19.1 ± 0.5 |
1.09 ± 0.09 |
46.7 ± 1.1 |
8.2 ± 0.4 |
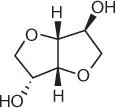
Figure 1.40 Chemical structures of isosorbide.
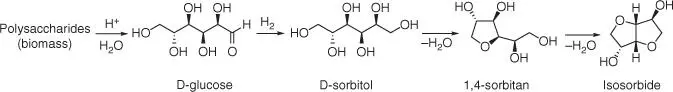
Figure 1.41 Schematic reaction pathway for the production of bio‐based isosorbide.
Isosorbide (1,4:3,6‐dianhydro‐D‐glucitol) is an organic oxygen‐containing heterocyclic compound composed of two fused furan rings ( Figure 1.40).
Isosorbide does not occur naturally. It can be obtained from various raw materials by organic synthesis through different reaction pathways. However, nowadays, isosorbide is produced from diverse polysaccharides through the multistep process that includes several intermediates [120] ( Figure 1.41).
The synthesis of isosorbide can start from polysaccharides (mainly starch or cellulose biomass) or directly from all of the intermediate compounds because they are currently commercially available on a large scale. Lignocellulosic biomass is considered as one of the best resource due to its abundancy, versatility, and price [121]. The synthetic process of isosorbide production from polysaccharides consists of the following stages: acid‐catalyzed hydrolysis of the glycosidic bonds in the polymeric carbohydrates, hydrogenation of obtained glucose to sorbitol and further dehydration to sorbitan, and finally dehydration of sorbitan to isosorbide. Moreover, the different side reactions (such as degradation or polymerization) can occur in this complex process. Therefore, different new synthesis strategies (including the one‐step synthesis from glucose and cellulose) and catalysts are still elaborated and proposed [122].
Isosorbide can be derivatized by functionalization or substitution of the two secondary hydroxyl groups present in its molecule. A certain difficulty is the different reactivity and steric hindrance of the hydroxyl groups (against, due to these, a selective monoderivatization is also possible). Nevertheless, isosorbide is currently converted to valuable derivatives for use as pharmaceuticals, detergents, emulsifiers for cosmetics, surfactants, stabilizers, or plasticizers. In addition to the above‐mentioned applications, for a long time, isosorbide is considered and intensively studied as a potential monomer building block for the biopolymeric materials (e.g. polycarbonates, polyurethanes, or polyesters). As the bio‐based diol with bicyclic rigid structure isosorbide can also be applied as a substitute for bisphenol A replacement, there are generally two ways for introducing an epoxy functionality into the isosorbide molecule ( Figure 1.42): conversion to the diallylic derivative, followed by oxidation to oxirane rings (the first pathway) and reaction with epichlorohydrin (the second and third pathways).
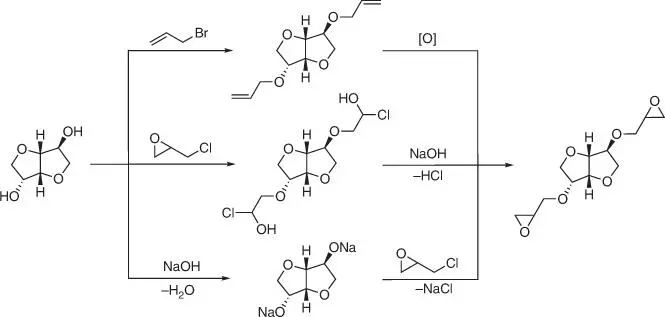
Figure 1.42 Possible reaction pathways for the synthesis of the diepoxide derivative of isosorbide.
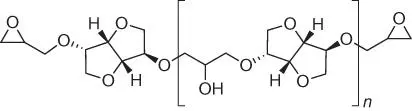
Figure 1.43 Structure of isosorbide‐based epoxy resins.
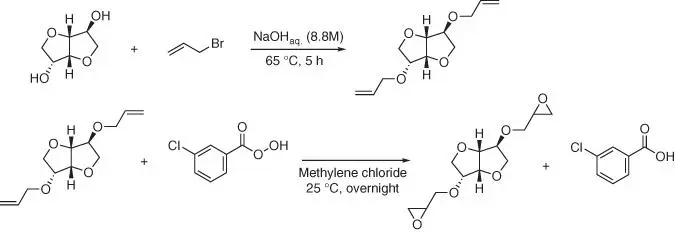
Figure 1.44 Synthetic route of diallyl isosorbide and isosorbide diglycidyl ether.
In fact, the chemical structure of the diglycidyl isosorbide derivative shown in Figure 1.42is valid only for the first reaction pathway. Only by the reaction with allyl bromide, followed by oxidation of the terminal unsaturated bonds, it is possible to obtain the product containing only one isosorbide molecule (the monomer). For the remaining reaction pathways, even the use of a large excess of epichlorohydrin leads to the formation of the products with an oligomer structure ( Figure 1.43).
According to the first reaction pathway, the diallyl ether can be prepared by heating the isosorbide with allyl bromide in sodium hydroxide solution ( Figure 1.44) [123].
Oxidation of diallyl isosorbide ether to isosorbide diglycidyl ether is carried out in the reaction with meta ‐chloroperbenzoic acid as an oxygen carrier. It is also possible to use 4‐allyoxybenzoly chloride to introduce the diallyl functionality ( Figure 1.45) [124].
Читать дальше



















