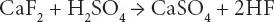6 A supply of fluorspar is maintained by the Defense National Stockpile Center. The Center sells the fluorspar as required.
7 The Montreal Protocol (1987) banned most chlorofluorocarbon (CFC) gases. A two-phase replacement plan was developed: 1. To transition from CFCs to Hydrochlorofluorocarbons (HCFCs) as an intermediate solution; 2. To replace HCFCs with Hydrofluorocarbons (HFCs) and 3. To replace all previous fluorocarbons with low Ozone Depleting and low Global Warming replacements such as hydrofluoroolefins.
8 Most CFCs were phased out by 1996; the rest were removed by 2010; HCFCs replaced CFCs. The replacement required a one-time large block of production to replace all fluids in refrigeration and air conditioning units in addition to other applications of CFCs.
9 European Union countries replaced all HCFCs with HFCs by the end of 2010.
10 In 2010 the Chinese Ministry of land and Resources placed a quota on the annual production rate of fluorspar limiting at 11 million tons.
11 In the recent years many programs have been implemented to capture and recycle fluorocarbons in addition to reducing consumption of these materials.
12 Some of the fluoropolymer manufacturing, specifically polytetrafluoroethylene, has moved away from the US, to China and India.
13 A reason fluorinated chemicals are more expensive than hydrocarbons is the cost of fluorspar.
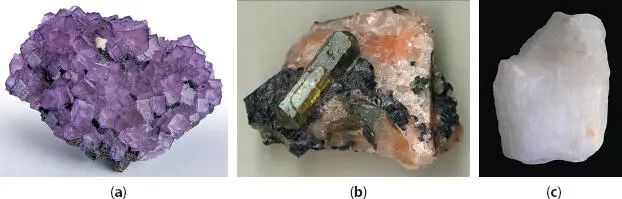
Figure 3.1Photographs of fluorine minerals: (a) fluorspar, (b) fluorapatite, and (c) cryolite Sources: (a) www.themineralgallery.com/elmwoodroom.htm, Courtesy : www.mindat.organd the Hudson Institute of Mineralogy (b) www.mindat.org/photo-16871.html, Courtesy : David Soler, (c) www.mindat.org/min-1161.html, Courtesy : JGW, May 2016.
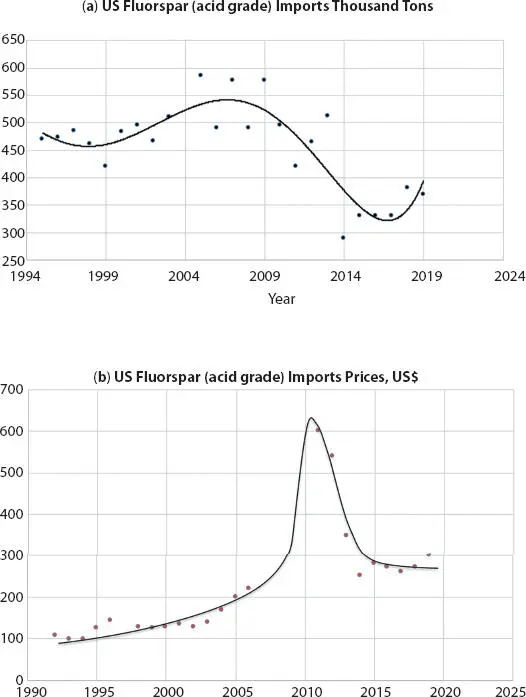
Figure 3.2Historical US imports and prices of acid grade fluorspar [7].
As shown in Figure 3.3, China mines more acid-grade fluorspar than any other country in the world, at 2,500 ktons annually. Mexico is the second-largest producer of acid-grade fluorspar at around 600 ktons annually, followed by South Africa (150 ktons). Contributions from the rest of the world have declined since the mid-2000s, with the remaining balance of acid-grade fluorspar production at about 300 ktons in 2016 [7].
From the mid-1990s to early 2000s, most of the acid-grade fluorspar mined in China was exported to the major refrigerant producing regions (US, EU, Japan). Starting from around 78% in 1999, the fraction of all mined acid-grade fluorspar in China that was exported has declined substantially. As a result of the dramatic expansion of the Chinese refrigerant and fluoropolymer manufacturing industry in the 2000s, most acid-grade fluorspar mined in China is now consumed domestically. Only 8% of the acid-grade fluorspar mined in China in 2016 was exported.
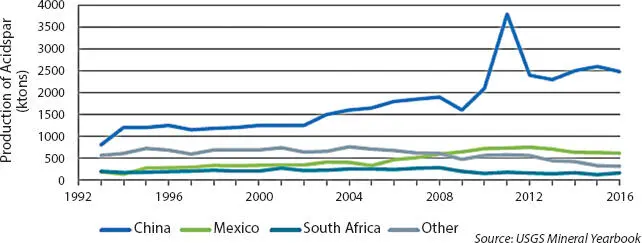
Figure 3.3Global mined quantities of acid-grade fluorspar during 1993 to 2016 [8].
In 2008, Mexico overtook China as the leading exporter of acid-grade fluorspar to the US and in 2009 became the largest fluorspar exporter globally. In 2016, Mexico supplied about 65% of the acid-grade fluorspar imported into the United States (see Figure 3.4) [8].
HF itself is also traded globally. it is, however, both toxic and corrosive making it difficult to transport great distances. Exported quantities are thus substantially lower than those of fluorspar, and HF export is mostly limited to nearby countries. For example, China primarily exports HF to Japan and South Korea, whereas nearly all Mexican HF exports go to the US. Likewise, most HF imported into the United States comes from Mexico (see Figure 3.5) [8].
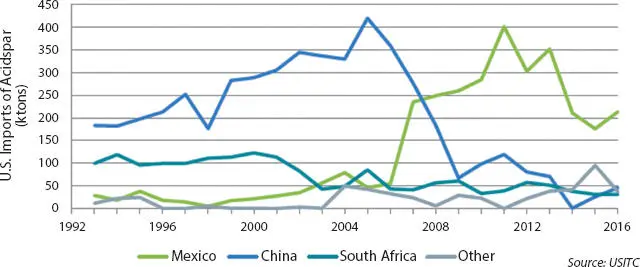
Figure 3.4Annual U.S. import quantities of acid-grade fluorspar (“acidspar”), 1993–2016. Mexico, China, and South Africa are the major countries of origin for acidspar. Other countries that have exported significant quantities of fluorspar to the United States include Vietnam, Spain, the United Kingdom, and Mongolia [8].
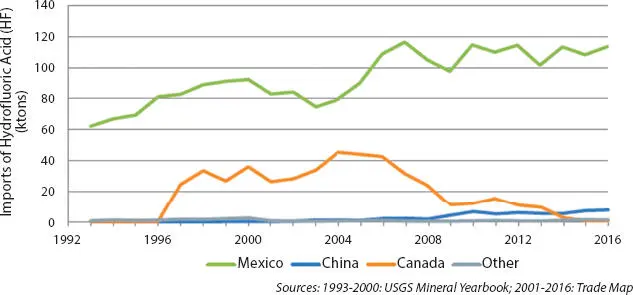
Figure 3.5U.S. imports of hydrofluoric acid, 1993–2016 [8].
3.3 Fluorocarbon Compounds
There are a number of routes to introduce fluorine into organic compounds [9–11]. Those methods include direct fluorination of carbon; halogen exchange between chlorocarbons and hydrofluoric acid; fluorination of hydrocarbons using electrochemical and catalytic methods; and fluorination techniques using metal fluorides [12]. Chapter 6 describes some of the commercial techniques for fluorocarbon preparation in detail.
The commercial manufacture of fluorocarbons requires converting fluorine’s inorganic ores to a suitable intermediate. That would in turn be used to introduce fluorine into organic compounds. A suitable compound would react with hydrocarbons (though not too reactive), inexpensive and is safe would be ideal. The most frequently used agent, commercially speaking, has proven to be hydrofluoric acid (HF), far from a perfect choice (Table 3.3).
HF when combined with water forms a highly corrosive acid that can even etch glass. Skin contact, inhalation, ingestion and contact with eyes must be avoided because of the extreme danger hydrofluoric poses. Safety data sheet (SDS) of HF must be consulted prior to its handling.
3.4.1 Manufacturing Hydrofluoric Acid
Hydrofluoric acid is manufactured by the reaction of acid-grade fluorspar (≥97% CaF 2) with sulfuric acid (H 2SO 4). The basic reaction is shown in Eq. ( 3.1).
(3.1) 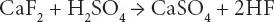
Figure 3.6 displays a process diagram for commercial production of HF [14]. In the first step fluorspar is dried for 30-60 minutes in a horizontal rotary kiln that is heated to 200-250°C. Dry fluorspar and a small excess of sulfuric acid are fed continuously to the front end of a stationary pre-reactor or directly to the kiln by a screw conveyor. The pre-reactor mixes the components prior to charging to the rotary kiln. Calcium sulfate (CaSO 4) is removed through an air lock at the opposite end of the kiln. The gaseous reaction mixture - HF and the excess H 2SO 4from the primary reaction are removed at the front end of the kiln along with entrained particulates. Silicon tetrafluoride (SiF 4), sulfur dioxide (SO 2), carbon dioxide (CO 2), and water produced in secondary reactions are also removed along with the HF.
Table 3.3Typical physical properties of hydrogen fluoride and hydrofluoric acid [13].
| Concentration |
49% HF |
70% HF |
100% HF (AHF) |
| Freezing Point |
-33°F (-36°C) |
-95°F (-71°C) |
-118°F (-84°C) |
| Boiling Point |
223°F (106°C) |
146°F (63°C) |
67.1°F (19.5°C) |
| Density (68°F) |
9.6 lbs/gal |
10.1 lbs/gal |
8.3 lbs/gal |
| pH |
<3.4 |
<3.0 |
<1.0 (10% solution) |
| Flash Point |
Not Flammable |
| Vapor Pressure (68°F) |
23 mm Hg |
132 mm Hg |
771 mm Hg |
| Vapor Density |
2.4 (air = 1.0) |
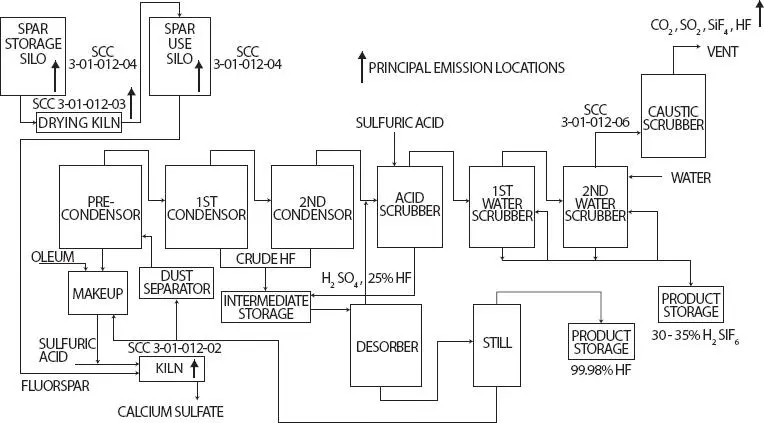
Figure 3.6Hydrofluoric acid manufacturing process flow diagram [14].
Читать дальше