Chemours Corp is the only commercial manufacturer of vinyl fluoride and its homopolymer polyvinyl fluoride (PVF). PVF cannot be processed by the standard melt processing method because it undergoes rapid thermal decomposition when heated above its melting point (195°C). PVF is fabricated into films and coatings using a somewhat unusual process. PVF is first dispersed in a latent (polar) solvent that suppresses its melting point thus permitting its melt processing without decomposition [16].
Fluoropolymer applications traverse across virtually every industrial segment and geographical regions. Examples of industries include automotive, aerospace, chemical and petrochemical processing, electrical, microelectronic, semi-conductor, pharmaceutical, biopharmaceutical, consumer, sports and recreation, food, beverage, laboratory applications, construction and architectural, military and others.
PTFE is the best material for manufacturing parts including gaskets, vessel and pipe linings, seals, spacers, dip tubes and other applications where corrosion resistance against most chemically challenging agents are required. Copolymers of TFE may also be used to manufacture some of those parts and wire and cable insulation. Most acids, bases and organic solvents do not affect these polymers even at elevated temperatures. PVDF applications include wire insulation, tubing and pipes for high purity water transportation, membrane distillation, gas separation, separator for lithium ion battery. PCTFE films are part of the composites for manufacturing bubble packs for pharmaceuticals because of their resistance to moisture vapor permeation.
Fluorocarbon elastomers are the largest group of fluoroelastomers. Different polymers are fluorinated to variable extents; most of fluorine is bonded to the carbon-carbon backbone of the macromolecule. Commercial fluoroelastomers are based on a number of monomers: tetrafluoroethylene (TFE), hexafluoropropylene (HFP), perfluoromethyl vinyl ether (PMVE), vinylidene fluoride (VDF), chlorotrifluoroethylene (CTFE), 1-hydro-pentafluoropropane (HPFP), ethylene, and propylene. Specific combinations of two or more of these monomers result in amorphous polymers with elastomeric behavior. A more complete list of monomers combined in significant commercial fluoroelastomers has been provided [17].
The first commercially available fluoroelastomer was Kel-F ®developed by the M.W. Kellog Co. in the late 1950’s. A well-known fluorinated elastomer was a copolymer of TFE and VDF developed by DuPont under the trade name Viton ®A. Later, Viton ®B was developed that was a terpolymer of TFE/VDF/HFP. Vinylidene fluoride based fluoroelastomers have been the most popular in the group. Fluorocarbon elastomers have grown extensively over time because of the need for high performance products.
Fluoroelastomers may be classified by their fluorine contents, 66%, 68%, & 70% respectively. Fluoroelastomers with higher fluorine content have higher fluids resistance due to higher fluorine content. Peroxide cured fluoroelastomers have inherently improved water, steam, and acid resistance. Fluoroelastomers are, generally, manufactured by emulsion polymerization process. The monomers are charged to a batch reactor and polymerized under elevated temperature and pressure in the presence of surfactants and additives. After polymerization has been completed the latex is discharged from the reactor, the polymer coagulated, washed, dried and prepared for shipping. Chemical formulas of major fluoroelastomers are shown below:
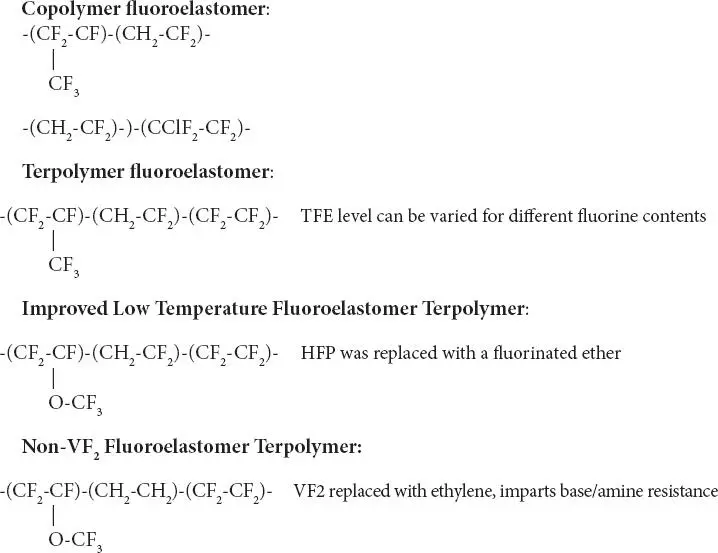
Fluoroelastomers provide high levels of resistance to chemicals, oil and heat, and service life at elevated temperatures, some exceeding 200°C. Fluoroelastomers are fabricated into a variety of shapes like hoses, seals, O-rings, shaft seals, diaphragms, vibration dampeners, expansion joints and electrical connectors and many others for demanding end uses in a number of industries:
Aircraft and aerospace
Automotive
Chemical processing and transportation
Oil and gas exploration and production
Petroleum refining and transportation
Pharmaceutical
Fluoropolymer coatings (also paints or finishes) have also developed based on their stable dispersions obtained by emulsion polymerization. The two coatings with the largest market share are based on polytetrafluoroethylene and polyvinylidene fluoride. One well-known application of these coatings is in non-stick cookware and bake ware. Fluorinated coatings are extensively applied to surfaces in industrial environments to protect them from a variety of corrosive agents [13].
Coatings have the advantage of ease of propagation based on few fluoropolymers by manipulating the formulation. Fluoropolymers can be converted into coatings in two separate ways, as liquids or solids. PTFE must be applied as a liquid coating in contrast to the melt processible fluoropolymers that can also be powder-coated. Fluoropolymer paints are formulated as water-based (aqueous) or solvent-based products. The latter is limited to cases in which aqueous dispersions would not work.
Fluorinated coatings, similar to other paints, are mixtures of a variety of components including liquid carriers, pigments and specific additives in addition to fluoropolymers and additional polymeric binders. Pigments affect the color and appearance of the coatings as well as its functions such as abrasion resistance, thermal conductivity, the extent of porosity and electrical resistance/conductivity. Specific additives are sometimes incorporated to modify the rheology of the paint, wettability of the final coating and facilitate formation of the film at the time of baking. Other polymers are included in the paint to enhance the adhesion of the paint to the substrate or increase its hardness thus overcoming shortcomings of fluoropolymers.
Like other paints those made with fluoropolymers can be applied as one or more “coats” or layers. Flexibility of fluoropolymer content and availability of thickness ranges (2.5-2,000 µm) are two important features of those paints. The base polymers polyvinylidene fluoride and polytetrafluoroethylene provide a broad range of bake temperature ranging from 175 to 440°C. The combination of material properties, coating thickness and process variables determine the performance parameters of the final paint.
Applications of fluorinated paints include chemical process equipment liners, insulating coatings for electronics, non-stick coatings for cookware, surgical patches and glass fiber fabric coatings used for roofing of large structures flue gas heat exchangers, interior of exhaust ductworks and others.
HFOs Hydrofluoroolefins (HFOs) are the fourth generation of fluorine-based gases. HFC refrigerants are composed of hydrogen, fluorine and carbon atoms connected by single bonds between the atoms. HFO refrigerants are composed of hydrogen, fluorine and carbon atoms but contain at least one double bond between the carbon atoms. HFO refrigerants have zero ODP and low GWP thus offer a more environmentally friendly alternative to CFCs, HCFCs and HFCs.
DuPont (Chemours) and Honeywell jointly developed HFO 1234yf which is sold under the brand names Opteon TMyf and Soltice ®yf. This low GWP fluorocarbon is also a replacement for R134a for use in mobile air conditioning (MAC) systems in the automotive sector.
1. Tavener, S.J. and Clark, J.H., Chapter 5: Fluorine: Friend or Foe? A Green Chemist’s Perspective, in Fluorine and The Environment , Vol. 2, A. Tressaud (ed.), Elsevier, 2006.
Читать дальше













