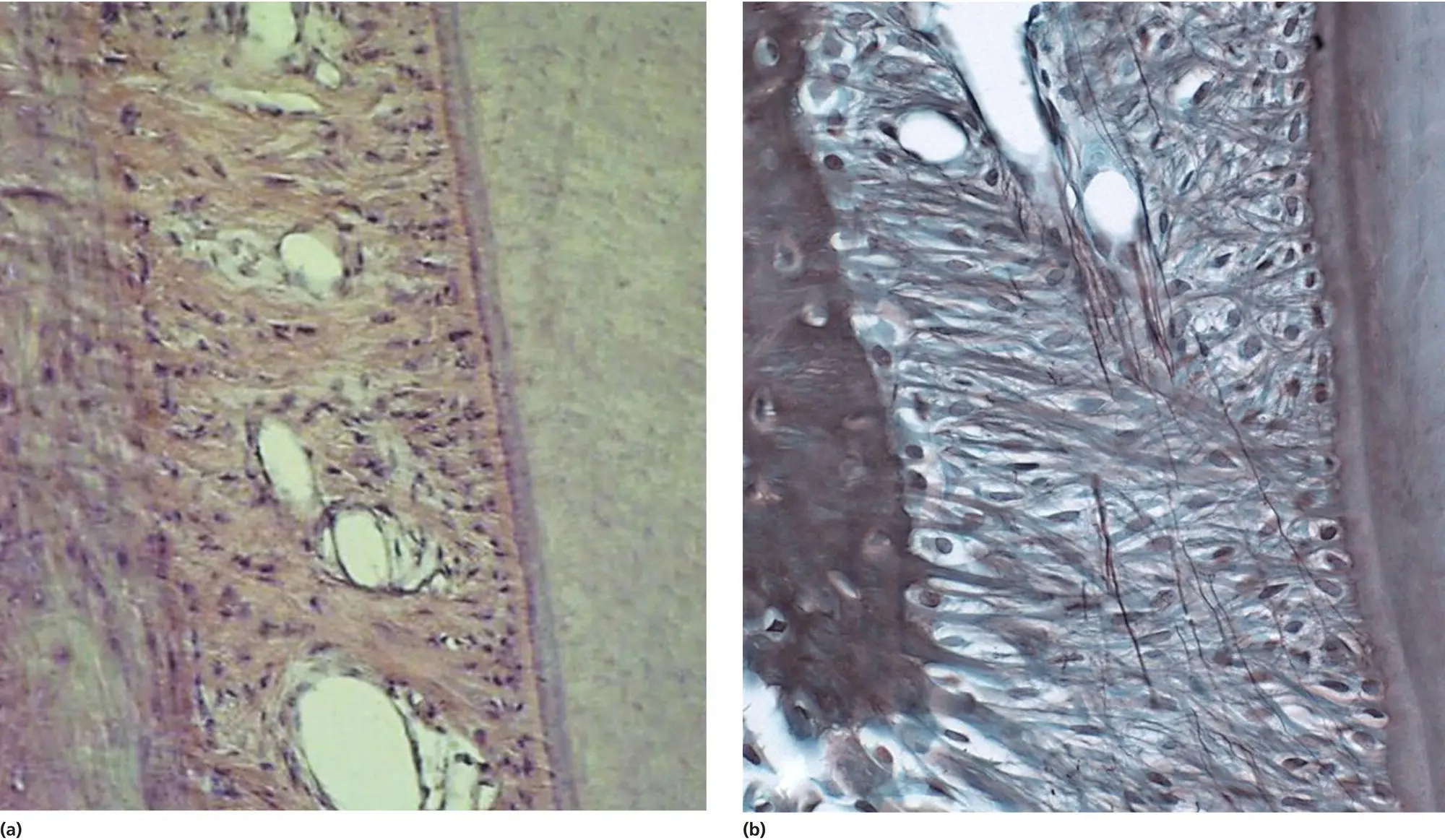
Figure 3.1 Photomicrographs of the normal PDL in a dog, showing the main orientation of the collagen fibers (A, H & E staining) and the oxytalan fibers (B, Oxone‐Halmi Aldehyde Fuchsin staining).
(Source: Jaap Maltha.)
A second type of fiber in the PDL is the oxytalan fiber ( Figure 3.1B). This type of fiber belongs to the elastic fiber family, which consists of elastic, elaunin, and oxytalan fibers. The elastic and elaunin fibers mainly contain elastin and fibrillins, while the oxytalan fibers lack elastin and only contain fibrillin‐1 and fibrillin‐2. These glycoproteins are synthetized in fibroblasts and polymerize after exocytosis, and, through lateral association and the incorporation of other components, they form microfibrils. Individual microfibrils again associate with one another to form microfibril bundles, the oxytalan fibers (Marson et al ., 2005; Hubmacher et al., 2006; Kielty, 2006; Strydom et al ., 2012).
The ground substance is primarily composed of water and large organic molecules, such as glycosaminoglycans (GAGs), including hyaluronic acid, heparan sulfate, dermatan sulfate, and chondroitin sulfate. Most of the GAGs are bound to proteins and then called proteoglycans. They are able to bind a considerable amount of water, giving the ground substance a gel‐like texture (Nanci and Bosshardt, 2006; Bergomi et al ., 2010; Ortun‐Terrazas et al ., 2018).
In the PDL, but also in all other connective tissues, the fibrous components are embedded in the “ground substance”, a network of proteoglycans, such as heparan sulfate, dermatan sulfate, and chondroitin sulfate, consisting of a core protein covalently bound to GAG chains. These GAGs can form large complexes when hundreds of GAG molecules become noncovalently attached to a single long polysaccharide molecule, such as hyaluronic acid. Under physiological conditions, GAGs have a strong water‐binding capacity as the GAG chains are negatively charged due to the presence of sulfate and uronic acid groups (Nanci and Bosshardt, 2006; Bergomi et al ., 2010; Ortun‐Terrazas et al ., 2018). They form an amorphous gel‐like structure, of which the stiffness depends on the amount of bound water. Apart from the bound water, also free water is present in the ground substance. The viscoelastic characteristics recorded for the PDL essentially result from interactions between unbound fluid and the compressible visco‐elastic porous matrix that makes up the bulk of the ground substance. Proteoglycans of the ground substance also bind to fibrous matrix proteins, such as collagen and oxytalan (Svensson et al ., 2001). Together, these components form the ECM, which acts as a substrate for PDL cells and allows them to migrate and to communicate with each other (Kerrigan et al ., 2000; Waddington and Embery, 2001; Nanci and Bosshardt, 2006; Dean, 2017; Listik et al ., 2019).
Finally, the PDL contains extensive vascular and neural systems. The blood vessels originate from three sources: apical vessels, which branch from vessels that supply the pulp; perforating vessels, which originate from the lamina and perforate the cribriform plate in the socket wall; and the gingival vessels, which come from the gingival tissue. Blood vessels in PDL may help in mechanical suspension and support of the tooth and supply surrounding PDL. These vessels transport blood cells, nutrients, and oxygen to the tissues of the PDL and remove waste and carbon dioxide (Lee et al ., 1991; Selliseth and Selvig, 1994; Dean, 2017)
The neural system in the PDL contains free and specialized nerve endings. Free nerve endings are nociceptive and are present along the whole length of the tooth. The specialized nerve endings are divided in Ruffini‐like endings, coiled nerve endings, spindle‐shaped nerve endings, and expanded nerve endings. The Ruffini‐like endings are mainly present near the root apex and secrete various neuropeptides, such as calcitonin gene‐related peptide (CGRP) and substance P. The coiled type endings are mainly located in the mid‐region of the PDL. Both act as mechanoreceptors and as fast acting nociceptors (Maeda et al ., 1990; Davidovitch, 1991; Maeda et al ., 1999; Krishnan and Davidovitch, 2006; Yamaguchi et al ., 2012 Dean, 2017)
The fibroblast is the major cell type in all connective tissues, including the PDL. Fibroblasts differentiate from mesenchymal stem cells and are responsible for the synthesis and secretion of all the elements of the ECM, collagen and oxytalan fibers, and proteoglycans. Furthermore, fibroblasts synthesize and secrete a wide variety of regulatory molecules that act as local signals for the adaptation of the tissue to changing conditions (Lekic and McCulloch, 1996; Jiang et al ., 2016).
Osteoblasts are large cells (20–30 μm), in the form of a polyhedron, with a basophilic cytoplasm, and with a substantial rough endoplasmic reticulum and Golgi apparatus. They originate from the mesenchymal stem cells of the bone marrow, endosteum, periosteum, and perivascular pericytes (Fernández‐Tresguerres‐Hernández‐Gil et al ., 2006a). They differ from fibroblasts because they can express RUNX2, which is essential for the differentiation of mature osteoblasts. RUNX2 is the first transcription factor that is upregulated in pre‐osteoblasts and it is downregulated again in mature osteoblasts (Li et al ., 2018). The osteoblasts synthesize and secrete the collagen and noncollagen proteins, such as osteocalcin and osteopontin, that form the organic bone matrix or osteoid material. Furthermore, they express alkaline phosphatase (ALP), which is essential for the mineralization of the osteoid (Lerner et al ., 2019). Osteoblasts also produce and secrete hydroxyapatite into the osteoid, forming the strong and well‐organized mineralized matrix of the bone (Hasegawa, 2018). Part of the osteoblasts are buried in the bone matrix as osteocytes, maintaining contact with each other and osteoblasts through extended cellular processes that lie in narrow canals within the bone matrix, the canaliculi ( Figure 3.2).
Although osteocytes are relatively inert cells, they are capable of transmission of signals over long distances through the canalicular network (Lerner, 2012). They are considered to be mechanosensory cells that play an important role in the regulation of the activity of osteoblasts and osteoclasts (Burger and Klein‐Nulend, 1999; Klein‐Nulend et al ., 2013; Tresguerres et al ., 2020) ( Figure 3.3)
Osteoclasts are multinucleated cells that are derived from hemopoietic stem cells, and more specifically from extravasated monocytes that can differentiate into macrophages and through fusion into osteoclasts. They are responsible for the resorption of bone. The formation of an effective seal around the resorption compartment is essential, because it enables the formation of an isolated compartment between the bone and the cell, called Howship’s lacuna. The cell membrane of the Howship’s lacuna becomes highly invaginated and forms the so‐called ruffled border, allowing massive secretory and endocytotic activity. This enables the vesicular transcytosis of the mineral and degraded collagen from the ruffled border to the free membrane of the cell, and its release into the extracellular compartment (Roodman, 1993; Duong et al ., 2000; Fernández‐Tresguerres‐Hernández‐Gil et al ., 2006b; Takahashi et al ., 2007). For an elaborate overview on the concerted interplay between osteoblasts, osteocytes, and osteoclasts see Lerner (2012) and Chapter 4of this book.
Читать дальше