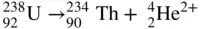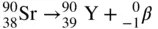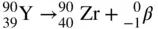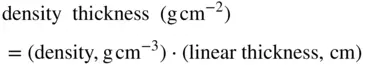1 ...6 7 8 10 11 12 ...42 Since radioactive isotopes are used because of their radioactivity, the unit for expressing the quantity of radioactive material is based on the disintegration rate. Thus, for example 3.7 × 10 10atoms in 1 g of 226Ra decay in 1 s, while 1.7 × 10 14atoms in 1 g of 210Po decay in 1 s. One gram of 210Po is much more radioactive than 1 g of 226Ra. Clearly, mass is not the most appropriate unit for specifying the quantity of a radioisotope. The traditional unit for the quantity of a radioisotope is Curie, symbolized by Ci. The Ci is defined as that quantity of a radioactive material in which 3.7 × 10 10atoms are transformed in 1 s. The commonly used subunits are the millicurie, mCi (10 −3Ci), microcurie, μCi (10 −6Ci), nanocurie, nCi (10 −9Ci), and picocurie, pCi (10 −12Ci).
In the SI system, the unit for the quantity of radioactivity is the becquerel (Bq). One becquerel is defined as that quantity of radioactive material in which one atom disintegrates in 1 s. Since the Bq is such a small quantity of radioactive material, the kilobecquerel (kBq) (10 3Bq), megabecquerel (MBq) (10 6Bq), and the terabecquerel (10 12Bq) are frequently used. 1 MBq = 27 μCi.
The concentration of radioactivity is called the specific activity. Specific activity may be designated in traditional units of curies per unit mass or unit volume. In the SI system, the analogous quantities are given in megabecquerel per kilogram or in megabecquerel per cubic meter.
The specific activity, in Ci/g of a carrier free radioisotope, is given by
(2) 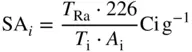
where T Raand T iare the half‐lives, in the same time units, of 226Ra ( T = 1600 years) and of the radioisotope, and A iis the atomic mass number of the radioisotope. In the case of 131 I , for example whose half‐life is eight days, the specific activity is
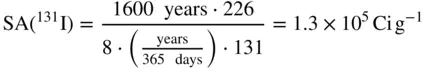
Radiation can be considered to be energy carried by one of several different kinds of subatomic particles. When these particles interact with living tissue they transfer their energy to the tissue. The energy that is absorbed by the tissue is dissipated in the disruption of atoms and molecules, thus leading to the biological effects associated with ionizing radiation. The particles of radiation are discussed below.
An alpha particle is a highly energetic nucleus of an ordinary helium atom, which consists of an assembly of two positively charged protons and two electrically neutral neutrons, and is designated by the symbol 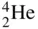 , that is emitted from the nucleus of certain radioactive isotopes. When an alpha particle is emitted, the alpha‐emitting parent is transformed into a daughter element whose atomic number is 2 less, and whose atomic mass number is 4 less than that of the parent. In the case of 238U, for example which is transformed to 234Th
, that is emitted from the nucleus of certain radioactive isotopes. When an alpha particle is emitted, the alpha‐emitting parent is transformed into a daughter element whose atomic number is 2 less, and whose atomic mass number is 4 less than that of the parent. In the case of 238U, for example which is transformed to 234Th
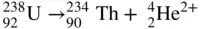
Two important properties of alpha radiation are the high rate of energy transfer to the medium through which the alpha particles travel and, consequently, a very small penetration range into an absorbing medium. Although most alpha particles from radioisotopes have kinetic energies in the range of 3–7 MeV, they can penetrate only a few centimeters of air before exhausting all their kinetic energy. Tissue and other solid materials are almost opaque to alpha particles. A 5.3‐MeV alpha particle from 210Po, for example whose range in air is about 4 cm, can penetrate only about 0.005 cm of soft tissue. This thickness is less than that of the dead outer layer of skin. For this reason, alpha radiation originating from alpha‐emitting radioisotopes outside the body is not considered hazardous. However, alpha radiation from an internally deposited radioisotope transfers its kinetic energy directly to viable tissue. The high concentration of absorbed energy, together with the microscopic distribution of the absorbed energy, makes internally absorbed alpha radiation more toxic per unit amount of absorbed energy than energy absorbed from the other radiations.
Beta particles are ordinary electrons that are ejected with a high kinetic energy from the nucleus of certain radioisotopes, resulting from the change of a neutron into a proton and the ejected beta particle. Emission of a beta particle results in the transformation of the beta emitter into another element whose atomic number is one greater than that of the parent, and whose atomic mass number remains unchanged. In the case of strontium‐90 ( 90Sr), for example a pure beta emitter, the daughter element is yttrium‐90 ( 90Y), the next higher element after Sr in the periodic table. In this case, the reaction does not stop with the production of 90Y. The yttrium daughter is also radioactive, and decays by beta emission to zirconium‐90 ( 90Zr), which is stable. These transformations are written symbolically as
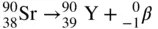
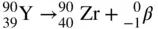
In the radioactive decay of many radioisotopes, gamma rays accompany the beta particles; these radioisotopes are called beta–gamma emitters. Radionuclides that emit only beta particles without any accompanying gamma radiation are called pure beta emitters.
Beta particles emitted by any particular radioisotope have a spread of energies, or a spectral distribution, that extends from near zero to a maximum that is characteristic of that radioisotope, as illustrated in Figure 1. The maximum energies vary from one radioisotope to another, and span a range of energies extending from several kiloelectron volts to several megaelectron volts. The average energy beta from any particular radioisotope is, in most cases, approximately one‐third of the maximum energy.
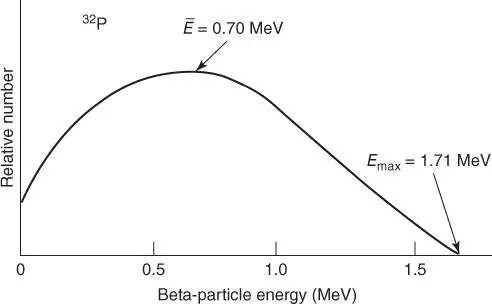
FIGURE 1
Beta energy spectrum for 32P.
Source: Based on data from Radiological Toolbox v. 1.0.0.
The depth of penetration, or the range of the beta radiation in matter, increases as the energy of the radiation increases. In air, very low‐energy betas have a range of several centimeters, while high‐energy betas travel about 3 m in air per MeV of energy. Figure 2shows the relationship between range and energy for beta particles.
The range of the beta radiation in Figure 2is expressed in units of density thickness . Density thickness is related to linear thickness by
(3) 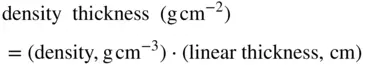
Читать дальше

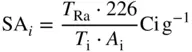
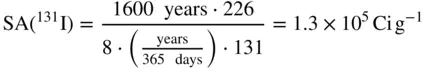
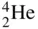 , that is emitted from the nucleus of certain radioactive isotopes. When an alpha particle is emitted, the alpha‐emitting parent is transformed into a daughter element whose atomic number is 2 less, and whose atomic mass number is 4 less than that of the parent. In the case of 238U, for example which is transformed to 234Th
, that is emitted from the nucleus of certain radioactive isotopes. When an alpha particle is emitted, the alpha‐emitting parent is transformed into a daughter element whose atomic number is 2 less, and whose atomic mass number is 4 less than that of the parent. In the case of 238U, for example which is transformed to 234Th