Accelerators. Intense beams of all radiation types are produced for medical radiation therapy, radioisotope production, neutron production for neutron activation analysis, and research.
Radioisotopes. This category includes sealed sources (for industrial uses such as gauging, inspection, food and drug sterilization, and medical radiation therapy), sealed neutron sources (for inspection, neutron activation analysis, and oil‐well logging), and unsealed radioisotopes (used in medical research, diagnosis, therapy, and generally in scientific and industrial research).
Nuclear reactors. Properly operating nuclear reactors produce negligible environmental radioactivity and exposure to the public. Nuclear reactors produce large quantities of neutrons in the fuel, resulting in the activation of many components in close proximity to the fuel. The fuel will contain both alpha‐ and beta‐emitting nuclides.
Technologically enhanced natural radioactivity. The concentration of radioactivity may be technologically increased to produce the so‐called TENORM (technologically enhanced naturally occurring radioactive material). Since this natural radioactivity is found in water and oil, in addition to rocks, coal, and soil, any scale that builds up in pipelines used for oil and gas production or for carrying water and brine in industrial facilities concentrates the radioactivity, especially radium. This can lead to significantly increased radiation exposure and inhalation hazards to workers when the pipelines are cut and the scale is dispersed as a radioactive aerosol.
Another source of TENORM is the phosphate industry. Phosphorous is usually associated with uranium because the two elements bind tenaciously together. Uranium concentrations of about 20–300 ppm are found in phosphate rock. Radiation from phosphate slag (which is used as an aggregate in road‐paving materials), phosphate fertilizers, and potash (which also contains relatively large amounts of 40K), thus must be considered by the industrial hygienist when evaluating possible occupational health risks in these industries.
Radioactivity is also found in relatively high concentrations in the fly ash and in the bottom ash of coal‐ and lignite‐burning boilers. Through this mechanism, many coal‐ and lignite‐fired electricity generating stations release more radioactivity to the environment than do nuclear power stations.
Workers who are explicitly involved with the use and maintenance of these radiation sources are not the only ones of concern to the industrial hygienist. The sources, once produced, must be transported from the manufacturer to the consumer. Additionally, radioactive waste is generally generated during the manufacture of the sradioisotopes as well as after they have been used. Thus, one must also apply the appropriate radiation safety standards and procedures to those workers involved in the transport of the radioisotopes and in the handling of waste materials.
3 BASIC PHYSICAL PRINCIPLES
3.1 Energy
All radiation effects, whether beneficial or detrimental, as well as all radiation measuring devices, are based on the absorption of energy from the radiation source.
In health physics and in atomic and nuclear physics, energy is measured in units of electron volts (eV). One electron volt represents the amount of kinetic energy possessed by an electron after it is accelerated by an electrical potential of 1 V. Commonly used multiples of the electron volt are the kiloelectron volt (keV), 1000 eV and the megaelectron volt (MeV), 10 6eV. One electron volt = 1.6 × 10 −12erg or 1.6 × 10 −19J; 1 MeV = 1.6 × 10 −6erg or 1.6 × 10 −13J.
The Bohr atomic model describes the atom as a positively charged central nucleus surrounded by electrons that revolve around the nucleus in specific radii, and are bound to the nucleus by the attractive electrical force between the positively charged nucleus and the negatively charged electron. The nucleus contains positively charged protons and electrically neutral neutrons. The attractive nuclear force between neutrons and protons and between neutrons acts to overcome the repulsive forces among the positively charged protons.
The atomic number, and hence the chemical nature of the element, is determined by the number of protons within the nucleus. While the number of protons may remain constant, most of the naturally occurring elements consist of nuclei that have differing numbers of neutrons. Nuclei of the same element that have different numbers of neutrons are called isotopes. Copper, for example whose atomic number is 29, is a mixture of two isotopes. About 69% of Cu atoms contain 34 neutrons, and hence has 63 particles (nucleons) within its nucleus, and about 31% have 36 neutrons, or 65 nucleons in the nucleus. The number of nucleons is called the atomic mass number. Symbolically, isotopes are written with the chemical symbol and the atomic mass number as a superscript to the left of the symbol: 63Cu and 65Cu in the case of copper 63 and copper 65, respectively. For an isotope to remain stable, the neutron/proton ratio must lie within a relatively narrow range. If the ratio is outside this range, the nucleus is unstable and achieves stability through a spontaneous nuclear transformation that corrects the neutron/proton ratio. Such an unstable isotope is called a radioisotope. Radioisotopes emit various combinations of particles and gamma rays that are specific to each isotope; this process is called radioactive transformation or radioactive decay. In most cases, the transformed nucleus is a different element, one whose neutron/proton ratio is closer to stability than the unstable parent's nucleus.
All radioisotopes are uniquely identified by a set of three different characteristics:
Type of radiation that is emitted
Energy of the emitted radiation
Rate at which the radioisotope decays. This rate is described either by the half‐life, or by the fraction that decays per unit time (the decay rate constant).
The half‐life is defined as the period of time during which one‐half of the radioisotope decays. After a second half‐life period, one‐half of the remaining isotope decays, leaving one‐fourth of the original activity, and after the next half‐life, only one‐eighth of the original activity is left. More than 99% of the original activity is gone after seven half‐lives. Half‐lives of radioisotopes range from microseconds to billions of years.
The decay rate constant is defined as the instantaneous fractional decrease of the radioisotope. In the case of 226Ra, whose half‐life is 1600 years, the decay rate constant is 0.00043 per year. This means that it is decreasing at a rate of 0.043% per year. Radioisotopes with a short half‐life decay at a much faster rate. For example, 131I, with a half‐life of eight days, decays at a rate of 8.7% per day. Generally, the relationship between an initial amount of activity, A 0, and the amount of activity left after a time t , and whose decay rate constant is λ per unit time, is given by
(1) 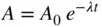
The half‐life, T 1/2, is related to the decay rate constant by
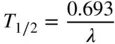
The half‐time, T 1/2, and the decay constant, λ , must be in the same time units when calculating with these decay equations.
3.4 Quantity of Radioactivity
Читать дальше