The polymer host is a long chain of a polymer having an electron-rich group (oxygen, nitrogen) in the chain. These coordinating sites provide the path to the ion migration after the application of the electric field. The flexibility and number of sites influence the ion migration as well as salt dissociation.
Properties of Salt
The addition of suitable salt is important as it directly affects the ion migration. For better electrical properties, salt must be dissociated completely in the cation and anion. The salt interacts with the electron-rich group of the polymer chain as well as with hydrogen in the polymer backbone. This results in the availability of free ions that contributes the conductivity. The important properties of the salt are lattice energy, cation radii, and non-toxic nature. The low lattice energy suggests the easier salt dissociation, and hence more free charge carriers while smaller cation radii favor the faster migration via coordinating sites. Also, the ionic conductivity and the voltage stability window of the salt needs to be checked first. In general, smaller cation radii and larger anion radii salt are chosen for optimum electrical properties.
Properties of Solvent
Both polymer host and salt are to be added in some solvent for developing polymer salt matrix. So, the solvent needs to have high dielectric constant, low viscosity, inert toward device components. The polymer chain interacts with salt and gets stretched insolvent that makes it easier for an ion to interact with the polymer chain. The high dielectric constant favors the better salt dissociation and also suppress the ion-pair formation.
Properties of Ionic Liquid
Ionic liquids (ILs) are molten salts (of three types aprotic, protic and zwitter) with bulky anion that improves the salt dissociation in the polymer matrix. The ionic radii of anion influence the electrical properties of the polymer matrix. Also, by changing the cation and anion structure, IL can be modified as per requirement.
Properties of Plasticizer
The incorporation of the low molecular weight plasticizer (EC, DEC, PEG, DMF) is an innovative approach to suppress the polymer crystallinity and enhance the ionic conductivity as well as flexibility (i.e., low T g; glass transition temperature). The plasticizer penetrates the polymer chains and reduces the cohesive forces between polymer chains which leads to enhanced segmental motion of the polymer chain. The enhanced segmental motion and improved free volume collectively enhance the ionic conductivity.
Properties of Nanofiller
Nanoparticles addition modifies the properties of the host matrix. So, the addition of nanoparticles of different morphology (spherical, wire, rod) has been examined for the development of suitable polymer electrolytes. The addition of nanoparticles enhances the salt dissociation due to surface group on the surface. The Lewis acid-base type indications with the salt and polymer results in enhanced polymer flexibility and more free charge carriers. The surface area is linked with the morphology of the nanofiller. The road and wire morphology provides a higher surface area for interaction and demonstrates faster ion migration than spherical morphology. The oxygen in the surface group (--OH) of nanofiller also provides additional conducting sites for ion migration along with sites provided by polymer chains. The nanoclay addition is also a beneficial approach and nanoclay with high cation exchange capacity is effective in enhancing ion dynamics. The nanoclay having a negative surface charge on clay layers allows polymer penetration inside it and accommodates cation coordinates polymer chain inside. It allows the cation migration by stopping the anions outside clay gallery owing to large anion size. It also suppresses the ion-pair formation tendency.
3.1.4 Modification Strategies for Polymer Electrolytes
Depending upon the addition of ionic liquid, plasticizer and nanofiller polymer electrolytes are categorized in three types, (i) ionic liquid-based polymer electrolyte, (ii) gel polymer electrolyte and (iii) solid/composite polymer electrolyte. The ionic liquid-based polymer electrolytes have polymer salt matrix with ILs incorporated in it. The IL increases the polymer flexibility and helps in salt dissociation. The g el polymer electrolytes (GPE) consist of plasticizer (EC, PC, DMF) in the polymer salt matrix. This type provides improved shape flexibility, enhanced electrical property and safety as compared to the ionic liquid. It enables the simultaneous presence of polymer matrix cohesive properties and liquid electrolytes type ion diffusion. The solid polymer electrolyte (SPE) is a solvent-free system and comprises polymer host, salt and nanofiller. The electron rich group in polymer matrix provides the coordinating sites for cation migration and nanofiller enhances salt dissociation along with modifying the polymer chain via Lewis acid-base interactions. The solid (dispersed) polymer electrolytes are prepared by the addition of inorganic insulating nanofiller (Active and passive) such as LiO, Al 2O 3, SiO 2, and TiO 2.
The high surface area with OH group of nanofiller effectively alters the polymer chain arrangement and also supports salt dissociation. The nanoparticle of various morphology such as nanorod, nanowire is effective due to higher surface area. The addition of nanoclay has also emerged as an attractive approach termed as a solid (intercalated) polymer electrolyte [30]. Figure 3.5shows the different types of polymer electrolytes. Table 3.2 shows some important polymer electrolytes and their characteristics.
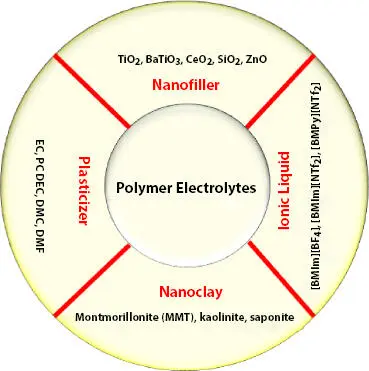
Figure 3.5 Types of additive based polymer electrolytes.
Table 3.2Properties of mostly used polymer host in polymer electrolytes.
| Abbreviation |
Polymer name |
Tg |
Tm |
Formula |
| PEO |
Poly(ethylene) Oxide |
-67 |
65 |
(-CE 2CE 2O-) n |
| PMMA |
Poly (methyl methacrylate) |
105 |
160 |
(C 5O 2H 8) n |
| PAN |
Poly(acrylonitrile) |
125 |
317 |
(C 3H 3N) n |
| PVA |
Poly(vinyl alcohol) |
85 |
230 |
(C 2H 4O) |
| PVC |
Poly(vinyl chloride) |
81 |
160 |
(C 2H 3Cl) n |
| PDMS |
Poly(dimethylsiloxane) |
-127 |
-40 |
-[SiO(-CH 3) 2]n |
| PVdF |
Poly(vinylidene fluoride) |
-40 |
171 |
-(CE 2CF 2)n- |
| PVdF-HFP |
Poly(vinylidene fluoridehexafluoropropylene) |
-65 |
135 |
-(CE 2CF 2) n[CF 2CF(CF 3)] m- |
| PEMA |
poly (ethyl methacrylate) |
66 |
160 |
[CH 2C(CH 3)(CO 2C 2H 5)] n |
3.2 Preparation and Characterization Techniques
The preparation of polymer electrolytes (PE) and characterization is an important part. The polymer electrolytes are prepared by solution cast technique, in-situ polymerization, Phase separation/inversion method, electrospinning technology, spin coating, melt intercalation, and hot press method. The structural and morphological examination is done by X-ray diffraction, Field emission scanning electron microscopy, and transmission electron microscopy. The electrical properties of PE like ionic conductivity, voltage stability window, and ion/cation transport number are evaluated from the impedance spectroscopy technique. The thermal properties of the PE are examined by thermogravimetric analysis and differential scanning calorimetry. The characterizations are summarized in Table 3.3.
Читать дальше













