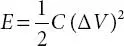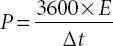Here I is the discharging current, Δ t is the discharge time, ΔV is the potential drop during discharge, and m is the total mass of the active electrode materials in the both (+ ve, - ve) electrode.
Energy Density & Power Density
(3.11) 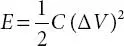
(3.12) 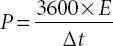
Here, E (Wh/kg), C, ΔV, P (W/kg) and Δt are the specific energy, specific capacitance, potential window, specific power and discharge time, respectively.
The recent development toward a suitable and safe supercapacitor has been achieved by developing various polymer electrolytes. Different polymer electrolytes have one common purpose to achieve high ionic conductivity, low crystallinity and hence high energy density/power density when used as the electrolyte in supercapacitor. This section reviews the significant research findings on various polymer electrolytes for supercapacitor applications. The incorporation of boron-containing segments in the polymer matrix appeared as a very attractive approach. Boron atom acts as an acidic center site due to an empty p-orbital and it supports the salt dissociation by interacting with the anion of electrolyte. Recently Jin et al . [36] prepared the polymer electrolyte (GPE) by incorporating the boron-containing segments in a rapid and easy one-step polymerization process assisted with UV light. The prepared GPE system was fully amorphous as confirmed from XRD. The highest conductivity 5.13 mScm -1was exhibited by Boron-containing GPE (B-GPE) at 25 °C and activation energy of 2.09 kJ/mol. The tensile stress for B-GPE was 1.30 MPa and the maximum strain was 62.8%. The B-GPE based all-solid-state supercapacitor shows the potential window of 3.2 V. The B-GPE based solid-state SC exhibits the specific capacitance of about 34.35 F/g (at 1 A/g). Figure 3.6shows the SC performance at various temperatures and specific capacitance increases from 21 to 74 F/g for temperature 0 to 80 °C (with voltage window 3.2 V). The energy density (54.20 Wh/kg) and power density (0.79 kW/kg) were higher ( Figure 3.6b, c). The SC cell demonstrates capacity retention of 91.2% after 5000 cycles ( Figure 3.6d).
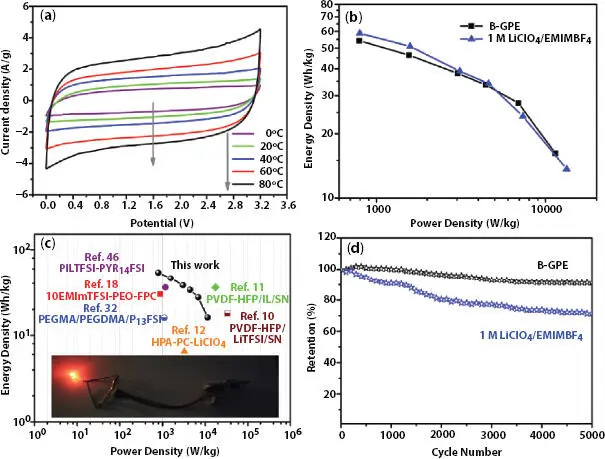
Figure 3.6 (a) CV curves of the all-solid-state supercapacitor with B-GPE under various temperatures from 0 °C to 80 °C at a scan rate of 50 mV s -1. (b) Ragone plots of the all-solid-state supercapacitor with B-GPE and conventional supercapacitor with 1 M LiClO 4/EMIMBF 4. (c) Ragone plots of the all-solid-state supercapacitor with B-GPE and others from previous articles for comparison (inset: photograph of a LED light powered by the all-solid-state supercapacitor with B-GPE). (d) The cycling performance of the all-solid-state supercapacitor with B-GPE and conventional supercapacitor with 1 M LiClO 4/EMIMBF 4at a current density of 1 A g -1. [Reprinted with permission from Ref. [36], © Elsevier 2011].
Another important strategy that is being focused on in research is the tuning of the electrode and electrolyte material. So to enhance the electrochemical performance of the SC cell, a novel approach was proposed by Du et al . [37]. They reported the fabrication of SC using poly(3,4-ethylenedioxythiophene/carbon paper (PEDOT/CP) as an electrode and gel polymer electrolyte [(1-butyl-3-methylimidazoletetrafluoroborate)/polyvinyl alcohol/ sulfuric acid (IL/PVA/H 2SO 4)]. The highest specific capacitance was 86.81 F/g at 1 mA/cm 2and capacity retention of 71.61 after 1000 cycles. The energy density values are also high and are 176.90 Wh/kg and power density is 21.27 kW/kg. The strong crosslinking points are generated by the Freezing–Thawing (F/T) method and PVA hydrogels are prepared. The number of F/T cycles is crucial. The specific capacitance increased with the increase of F/T cycles (upto F/T 3) and is 53.73 F/g and then decreases. The increase of F/T cycles increases the number of H-bonds in polymer gel and a 3D crosslinking network is formed that allows easier access to ion migration ( Figure 3.7c) [38].
Recently Alexandre et al . [39] reported the preparation of highly adhesive/sticky PIL/IL gel polymer electrolytes for application in solid-state supercapacitor. The GPE comprises poly(ionic liquid) consisting of poly(1-vinyl-3-propylimidazolium bis(fluorosulfonyl) imide) (poly(VPIFSI)) and a commercial ionic liquid: 1-ethyl-3-methyl imidazolium bis(- fluorosulfonyl)imide (EMIFSI). The prepared SC cell MWCNT||PIL/IL-GPE||MWCNT was examined in the voltage window of 2.5 V and the CV curve depicts EDLC nature. The specific capacitance was 9.6 F/g and coulombic efficiency was 94% ( Figure 3.8b). The energy density and power density for the cell vary from 8.8 Wh kg -1and 268Wkg -1to 4.6Wh kg -1and 3732Wkg -1, respectively.
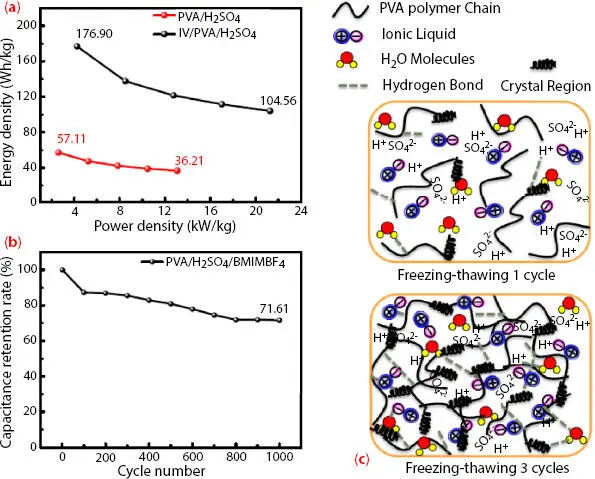
Figure 3.7 Ragone plot of the SSC based on two GPEs (a) and the capacitance retention rate of SSC-IL/ PVA/H 2SO 4(b) at 5 mA/cm 2, (c) The structure illustration. [Reproduced with permission from Ref. [37], © MDPI 2020].
Recently, polyelectrolyte (PE) based GPE is being investigated due to their superior electrochemical properties when used in SC application. The high water retention ability of PE provides conductive ion migration channels for ions in electrolyte [40]. So, Yan et al . [41] prepared the PE material by the UV-assisted copolymerization of a novel aprotic monomer N-(2-methacryloyloxy) ethyl-N,N-dimethylpropanammonium bromide (C 3(Br) DMAEMA) and poly (ethylene glycol) methacrylate (PEGMA). The conductivity of the PGPE was 66.8 Scm -1at 25 oC and the activation energy was 16.09 kJ/mol. The CV curve of the SC cell was almost rectangular and a specific capacitance of 64.92 F/g was observed at 1 A/g and 67.47 F/g at 0.5 A/g. The capacity retention was 84.74% at 0.5 A/g. The flexibility of the device was tested at different deformation rates. The SC cell shows an energy density of 9.34 Wh/kg and power density 2.26 kW/kg. The cyclic stability was also good and capacity retention was 94.63% after 10000 cycles at 2 A/g.
Another important electrolyte category is ‘redox-active electrolytes’ and are prepared by the addition of redox additives like methylene blue (MB), Iodide salts (KI, NaI), hydroquinone (HQ), p-diphenylamine, etc. [42]. Yadav et al . [43] prepared a GPE using 1-butyl-3-methylimidazolium bis(trifluoro-methylsulfonyl)imide (BMITFSI) as IL, and sodium iodide (NaI) as redox additive with poly(vinyledeneflouride-co-hexaflouropropylene) (PVdF-HFP) as host polymer. The specific capacitance was 351 F/g at 5 mV/s for SC cell with redox additive and is higher than redox additive-free cell (128 F/g) ( Figure 3.9a). The specific energy was 26.1 Wh/kg and power density of 15 kW/kg ( Figure 3.9b). Inset shows the LED glow demonstration for 300 s by connecting 4 cells. The SC cell with redox additive demonstrates superior cyclic stability (5% initial fading) than redox additive-free SC cell (23% initial fading) for 10000 cycles.
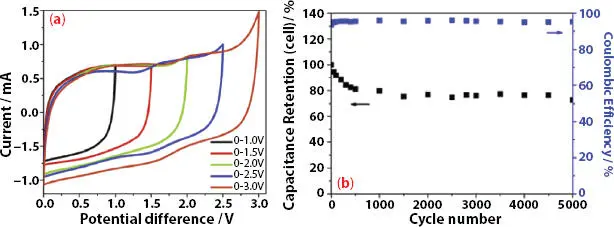
Figure 3.8 (a) Cyclic voltammograms obtained at 50 mV/s, (b) Capacitance retention as a function of the number of cycles at the operating voltages of [Reproduced with permission from Ref. [39], © Elsevier 2019].
Читать дальше