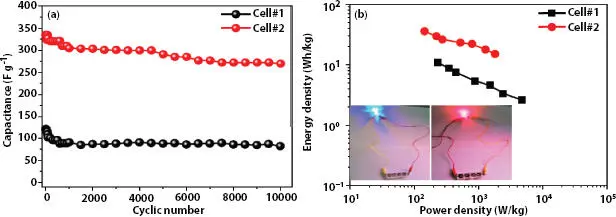
Figure 3.9 (a) Specific capacitance of Cell#1 and Cell#2 versus charge–discharge cycles measured at constant current density 0.84 A g -1, and (b) Ragone plots of Cell#1 and Cell#2 (inset shows glow of LED by four cells connected in series). [Reproduced with permission from Ref. [43], © Wiley 2019].
Another crucial approach to increase the electrochemical performance of SC cell is by developing composite materials (iongels) having two networks based on polarity [44]. Here, strongly polar (e.g., PEO, PVA, etc.) network provides superior electrochemical properties while, less polar network (e.g., NBR, natural rubber, PDMS, etc.) leads to enhanced mechanical properties. Based on this, Lu et al . [45] reported the preparation of iongels composite of PEO/NBR by in situ synthesis. The tensile modulus was 0.69 MPa and elongation break about 338% for iongel. The ionic conductivity was 2.4 mS/cm for 60% uptake of IL. Then using PEO/NBR iongels, an SC cell was fabricated using graphene electrodes and tested in the voltage window of 0-2.5 V. The specific capacitance was 2.8 F/g at 1A/g and decreases to 150 F/g at 10 A/g. The SC cell demonstrates good cyclic stability up to 10000 cycles (93.7% capacity retention) and negligible structural degradation as evidenced by XRD (after 10000 cycles). The energy density was very high 181 Wh/kg (comparable to commercial Lithiumion batteries) with a power density of 5.87 kWh/kg [46].
In solid polymer electrolytes (SPE) dielectric constant of the nanofiller also plays an important role in the enhancement of the ion transport parameters and hence the storage capacity of SC cell. So, to examine this Das et al . [47] investigated the effect of TiO 2(dielectric constant: 80) and ZnO (dielectric constant: 8.5) on polymer matrix of PVDF–HFP incorporating 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIMBF 4) as IL. The ionic conductivity of the prepared solid polymer electrolytes was 1.68 × 10 −2S/cm (nanofiller free), 2.57 × 10 −2S/cm (with ZnO) and 3.75 × 10 −2S/cm (with TiO 2) at 303 K. The electrochemical stability window of solid polymer electrolytes was 4.57 V (nanofiller free), 5.55 V (with ZnO) and 5.98 V (with TiO 2).
The ionic conductivity and voltage stability window were superior for TiO 2nanofiller based SPE. The specific capacitance for the SC cell [EDLC-I: nanofiller free, EDLC-II: with ZnO, EDLC-III: with TiO 2] using AC as electrode obtained from impedance spectroscopy was 103.5 F/g (nanofiller free), 134.6 F/g (with ZnO) and 206.4 F/g (with TiO 2). While specific capacitance for the SC cell from CV was 79.07 (nanofiller free), 93.07 (with ZnO) and 192.17 F/g (with TiO 2) at 25 mV/s. The increase in specific capacitance for TiO 2was attributed to a high dielectric constant which supports salt dissociation. The value of specific capacitance for the SC cell from GCD was 104 F/g (nanofiller free), 131 F/g (with ZnO) and 239 F/g (with TiO 2) at 1 A/g ( Figure 3.10a, b). The TiO 2based SC cell shows an energy density of 33.19 Wh/ Kg and a power density of 1.17 kW/Kg. Also, all cells demonstrate a coulombic efficiency of 100 % after 2000 cycles.
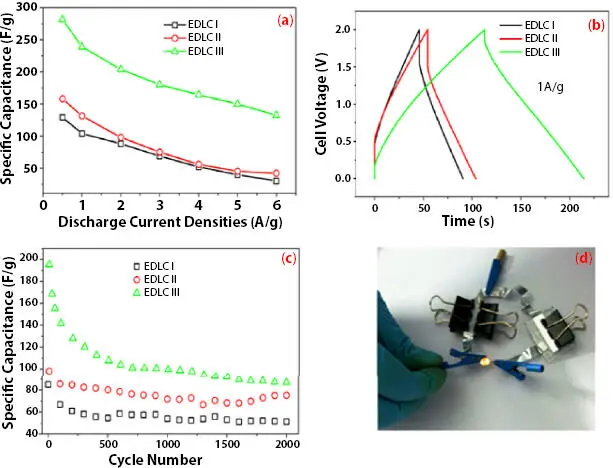
Figure 3.10 (a) Comparison of charge–discharge curves at current for EDLC I, EDLC II, and EDLC III at a current density of 1 Ag −1. (b) Variation of the specific capacitance of EDLC I, EDLC II, and EDLC III cells at different constant current densities. (c) Specific capacitance of EDLC I, EDLC II, and EDLC III cells at current density of 2 Ag −1shown as a function of charge–discharge cycles. (d) Two EDLC III cells in series to light up a yellow LED. [Reproduced with permission from Ref. [47], © Wiley 2020].
Another report from the same group examined the SC performance based on poly(vinylidene fluoride-co-hexafluoropropylene) polymer matrix, 1-propyl-3-methyleimidazolium bis(trifluromethylesulfonyl)-imide as ionic liquid with lithium bis (trifluoromethanesulfonyl)imide salt and plasticizer mixture (ethylene carbonate: propylene carbonate in the ratio 1:1) [32]. The highest specific capacitance from CV for cell 3 was 124.1 F/g at a scan rate of 10 mV/s with an energy density of 23.07 Wh/kg and power density of 0.5333 kW/ kg. Another report investigated the effect of cationic size and viscosity, dielectric constant of the ionic liquids on the electrochemical performance of SC cell [48]. Three polymer electrolytes were prepared: (i) BDMIMBF4-P(VdF-HFP) (BDMIMGPE-1), (ii) BMIMBF4-P(VdF-HFP) (BMIMGPE-2) and (iii) EMIMBF4-P(VdF-HFP) (EMIMGPE-3). The highest ionic conductivity was observed for the EMIMGPE-3 electrolyte and is 12.76 mS/cm which is attributed to the smaller cation size, high dielectric constant and low viscosity.
The value of specific capacitance as estimated from CV was 32.66 F/g (Cell-1), 49.1 F/g (cell-2), and 63.47 F/g (cell 3) at 10 mV/s. The highest specific capacitance for cell 3 is in correlation with ion conductivity results. The cell-3 demonstrates 74% capacity retention after 4000 cycles and 100% coulombic efficiency after 8000 cycles. The energy density and power density also superior to cell-3 ( Figure 3.11).

Figure 3.11 Ragone plots (gravimetric specific energy versus specific power) for all EDL cells. [Reproduced with permission from Ref. [48], © Elsevier 2018].
Recently, Choi et al . [49] reported a novel strategy to achieve long-term cyclic stability and high energy density. The authors used the nanofiber cellulose incorporated nanomesh graphene-carbon nanotube hybrid buckypaper electrodes and ionic liquid-based solid polymer electrolyte. The SC cell using cPT-200 polymer electrolyte demonstrates areal capacitance of 291 mF cm -2at a current density of 0.75 mA cm -2and capacity retention of about 96.3% after 50000 cycles at 7.5 mA/cm 2. Even after bending the SC cell, the capacity retention was 98.4% after 50000 cycles. The enhanced performance was attributed to the high ionic conductivity of polymer electrolyte (3.0 mS/cm) and high electrical conductivity of nanofiber cellulose incorporated nanomesh graphene-CNT hybrid buckypaper (540 S/cm). The SC cell exhibits gravimetric energy density: 33.6 Wh kg -1and volumetric energy density: 6.68 mWh cm -3.
Recently Jin et al . [50] reported the fabrication of SC cell using novel quasi-solid-state polymer electrolyte (QPE) of porous acrylate rubber/tetraethylammonium tetrafluoroborate-acetonitrile (pACM/Et 4NBF 4-AN) and nitrogen-doped porous graphene (NPG) film-supported vertically aligned polyaniline nanocones (NPG@PANI). The specific capacitance for NPG@PANI-2C electrode cell was 259.5 mF cm -2(330.2 F/g; 51.9Fcm -3) at 1mAcm -2( Figure 3.12a). The anode NPG@PDAA-3C was chosen and demonstrates specific capacitance of about 254.5 mF cm -2(294.4 F/g; 50.9 F cm -2) at 1mAcm -2( Figure 3.12b). Then an asymmetric SC device was fabricated using NPG@ PANI cathode, NPG@PDAA anode and pACM/Et 4NBF 4-AN as polymer electrolyte ( Figure 3.12c). The specific capacitance of asymmetric device was 6.2 F cm -3(124.7mF cm -2; 72.1 F/g) at 0.5 mAcm -2with 88.7% capacity retention after 10000 cycles. The energy density was 6.18mWh cm -3(123.5 mWh cm -2; 71.4 Wh/kg) with power density of 0.033Wcm -3(0.668mWcm -2; 0.386kW/kg).
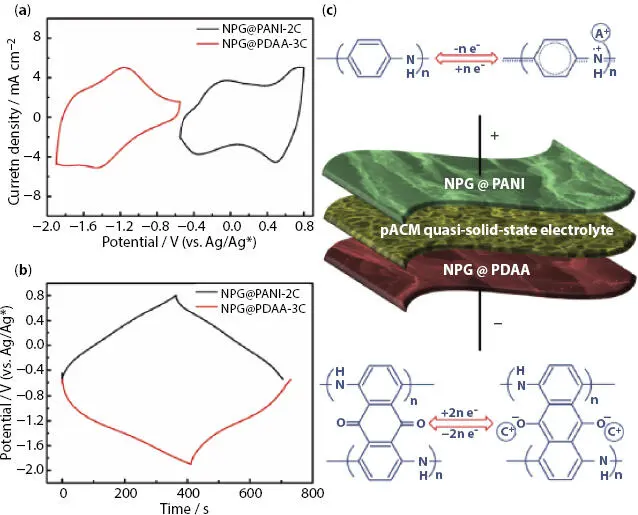
Figure 3.12 (a) CV curves at 10 mV s -1and (b) GCD curves at 1mA cm -2of NPG@PANI-2C and NPG@ PDAA-3C in three-electrode mode. (c) Schematic diagram of as-assembled NPG@PANI//QPE//NPG@PDAA oAFSC. [Reproduced with permission from Ref. [50], © Elsevier 2019].
Читать дальше
















