The ionic conductivity also varies with temperature. The increase of temperature thermally activates the charge carriers and lowers the activation energy or potential barrier required for ion migration. The variation of ionic conductivity with temperature follows three behaviors depending upon temperature range: (i) Arrhenius behavior, (ii) Vogel-Tamman-Fulcher (VTF) behavior, and (iii) Williams-Landel-Ferry (WLF) behavior [17–22].
Arrhenius behavior
The increase of temperature in the polymer matrix thermally activates the charge carriers and increase in flexibility leads to fast ion migration via coordinating sites. This collectively favors the ion dynamics and Arrhenius’s behavior suggests the ion transport occurs via hopping mechanism. This behavior dominates when the temperature is lower than the glass transition temperature (T g) [18]. To explore it further, activation energy is evaluated and the lower value of the activation energy is favorable for fast ion dynamics and hence promotes higher ionic conductivity. The activation energy ( Ea ) is slope of linear-least square fitting of the log σvs. 1/ T plot by Arrhenius equation and is expressed as; 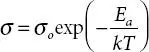 [Here, σ o is pre-exponential factor, k is Boltzmann constant].
[Here, σ o is pre-exponential factor, k is Boltzmann constant].
Vogel-Tamman-Fulcher (VTF) behavior
The VTF σ vs. 1/ T plot is the non-linear plot and ion transport occurs via the segmental motion of the polymer chain coupled with hopping. The ion diffusion within the polymer matrix occurs via the availability of free volume that is delivered by the polymer chains. The thermally activated charge carriers cross the potential barrier and contribute to conduction [19, 20]. The VTF equation is; 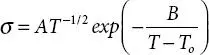 . Here, σ is the ionic conductivity, A is the pre-exponential factor, B is a constant, and T ois the temperature close to the T gof material (where entropy is zero).
. Here, σ is the ionic conductivity, A is the pre-exponential factor, B is a constant, and T ois the temperature close to the T gof material (where entropy is zero).
Cation/Ion Transference Number
As in polymer electrolytes, the main contribution is from the ion migration. So, the cation (t +) and ion (t ion) transference number is evaluated to check the exact contribution from ions and cation through cell configuration (SS|PE|SS), SS refers to stainless steel. The former is determined by a combination of ac impedance & d. c. polarization technique, while the latter is obtained from dc Wagner’s polarization technique [23, 24]. The cation transference number (t +) is obtained via relation; 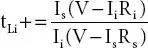 ; [V is the applied voltage across cell configuration, I iand I sare the initial and steady-state currents, R i, and R sare the interfacial resistance before and after polarization]. The ion (t ion) transference number is obtained via equation;
; [V is the applied voltage across cell configuration, I iand I sare the initial and steady-state currents, R i, and R sare the interfacial resistance before and after polarization]. The ion (t ion) transference number is obtained via equation; 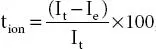 ; [I tand I eare the total current and the residual current respectively and are related as i t= i ion+ i elec].
; [I tand I eare the total current and the residual current respectively and are related as i t= i ion+ i elec].
Electrochemical Stability Window (ESW)
The voltage window of the electrolyte is a very significant parameter that needs to be examined before adopting polymer electrolytes for SC application. The energy density & capacity of the SC cell are directly linked with the voltage widow of electrolytes or devices. The voltage stability window is examined by the linear sweep voltammetry (LSV) technique which is plot of current vs. voltage. At any particular voltage onset of current occur and this is breakdown voltage or electrochemical stability window for that electrolyte.
3.1.3 Polymer Electrolytes and Types
Traditional energy storage devices use the liquid electrolytes and that restricts the shape geometry of the device and hence practical applications. The enhanced demand for flexible devices motivated the scientific community to search for suitable electrolyte material that can serve the purpose of flexible devices. So, polymer electrolytes emerged as a suitable candidate due to intrinsic flexibility in polymer and it completely matches with the need of future devices. The first report that mentioned the ionic conduction in polymer electrolytes was published by Wright and co-workers in 1973. It shows that the polymer host polyethylene oxide (PEO) mixed with alkali metal salts (NaI) demonstrates the enhanced conductivity than the pure polymer, and it opened the door for researchers to develop new material and explore the ion transport mechanism. Later on, in the early 1980s, Prof. Armand demonstrated the technological importance of the polymer electrolytes over existing electrolytes [25]. So, a polymer electrolyte comprises polymer and metal salt. This metal salt get dissociated in cation and anions by interaction with solvent and polymer chain. The electron rich group in polymer chain provides coordinating sites to cation for migration and cation jump from one coordinating site to another on application of filed (hopping mechanism). Anion due to large radii remains in immobilized state and hangs with the polymer backbone. Figure 3.4demonstrates the ion dynamics via hopping mechanism in polymer matrix [26].
The important component to the polymer electrolytes is the polymer host, salt, solvent, and nanoparticle. So, selection criteria need to be followed for developing suitable polymer electrolyte with optimum structural, electrical as well as mechanical properties [27]. In the following section, the important characteristics of the various components of the polymer electrolytes are discussed (Table 3.1) [16, 28, 29].
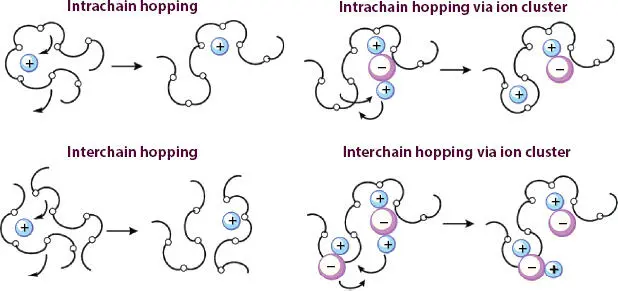
Figure 3.4 Mechanism of ion transport in PEO [Reproduced with permission from Ref. [26], © Royal Society of Chemistry 2015].
Table 3.1Fundamental characteristics of various constituents of the polymer electrolyte matrix. [Reproduced with permission from Ref. [16], © IOP Publishing 2017].
| Polymer Host |
Plasticizer |
| Low glass transition temperature (T g)High molecular weight, and Low ViscosityHigh degradation temperatureHigh Dielectric ConstantHave electron rich group (O, N) |
Low Melting Point and High Boiling PointHigh dielectric constant, and Low viscosityInert and cost-effectiveGood SafetyNontoxic Nature |
| Solvent |
Nanofiller |
| Abundant, and Non Aqueous in NatureLow Melting Point, and Low ViscosityLarge Flash PointHigh Dielectric ConstantGood Solubility for Polymer and Salt |
High Polarity, Low Melting, & High Boiling PointSafe, cost-effective and Non-toxicEnvironmental friendly, and Inert to All Cell Components.High Dielectric Constant for better salt dissociation |
| Salt |
Nanoclay |
| Low Lattice Energy, and High Ionic ConductivityHigh Mobility, and Broad Voltage Stability WindowSmaller cation radiiLarge Anion radiiHigh Thermal and Chemical StabilityLarge Transference NumberInert Towards Cell Components |
Layered/unique structure with high aspect ratio (~1000).Greater ability for intercalation and swellingHigh swelling index & High cation exchange capacity (CEC) (~80 meq/100 g)High external/internal surface area (~31.82 m 2g -1)Appropriate interlayer charge (~0.55)Adjustable hydrophilic/hydrophobic balance |
| Ionic Liquid |
Nanorod/Nanowire/Nanobelt |
| Good Thermal stability and broad Wide electrochemical stabilityLow Melting Point, and viscosityNegligible Volatility, Vapor PressureHigh Ionic ConductivityHigh Polarity, and High Dielectric ConstantNon-flammability |
High aspect ratioEasily alignment perpendicular to electrodesOxygen vacancies on the surface for cationLess agglomeration at high contentHigh thermal stabilityBroad voltage stability windowBetter chemical stability |
Polymer Host
Читать дальше

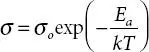 [Here, σ o is pre-exponential factor, k is Boltzmann constant].
[Here, σ o is pre-exponential factor, k is Boltzmann constant].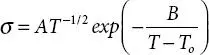 . Here, σ is the ionic conductivity, A is the pre-exponential factor, B is a constant, and T ois the temperature close to the T gof material (where entropy is zero).
. Here, σ is the ionic conductivity, A is the pre-exponential factor, B is a constant, and T ois the temperature close to the T gof material (where entropy is zero).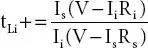 ; [V is the applied voltage across cell configuration, I iand I sare the initial and steady-state currents, R i, and R sare the interfacial resistance before and after polarization]. The ion (t ion) transference number is obtained via equation;
; [V is the applied voltage across cell configuration, I iand I sare the initial and steady-state currents, R i, and R sare the interfacial resistance before and after polarization]. The ion (t ion) transference number is obtained via equation; 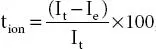 ; [I tand I eare the total current and the residual current respectively and are related as i t= i ion+ i elec].
; [I tand I eare the total current and the residual current respectively and are related as i t= i ion+ i elec].











