The present chapter describes the important development in the field of polymer electrolytes of application in the supercapacitor. The characteristics of polymer electrolytes will be discussed; then the selection criteria for the polymer, salt and additives will be the focus of discussion, followed by the classification of polymer electrolytes.
3.1.1 The Basic Principle and Types of Supercapacitors
A supercapacitor is an electrochemical device and is used to store energy and lies between the traditional capacitors and battery. The key advantages of SC are high power density, fast-charge discharge, high-performance stability, and long cyclic stability/life (∼10 6cycles). Along with these features, only one drawback needs to be explored, the low energy density of SC. A lot of research efforts have been demonstrated to increase the energy density of SC by tuning the electrode material, electrolyte, and device structure. Figure 3.1ashows the history of the supercapacitor worldwide [6]. The supercapacitor performance is influenced by the electrode material, electrolyte, and separator. These are further linked to the performance parameter of the SC cell examined by different characterization techniques. Figure 3.1bshows the schematic diagram which highlights the relation between different performance metrics, the major affecting factors, and the corresponding test methods. For clarity and good visibility to readers, several color schemes are employed. Three core parameters are highlighted in yellow; the power and energy densities in dark blue; time constant and cycling stability in light orange; all the important affecting factors in light purple; and the corresponding test methods in white [7].
The supercapacitor is different from the traditional capacitor or electrostatic capacitors as shown in Figure 3.2a. Depending on the charge storage mechanism, electrode material, electrolyte, and cell design are classified into three types. SC store energy and charge storage phenomena is an important criterion that decides SC performance. On the basis of the charge storage mechanism, SC is of three types [8]:
Electric double-layer capacitors (EDLCs), where the capacitance is produced by the electrostatic charge separation (no charge transport between electrode and electrolyte) at the interface between the electrode and the electrolyte ( Figure 3.2b). To maximize the charge storage capacity, the electrode materials are usually made from highly porous carbon materials for achieving
1 (1) maximum internal surface area. The charge absorption capability is generally 0.17-0.20 electrons per atom at an accessible surface [9, 10].
2 (2) Pseudocapacitors, which rely on fast and reversible faradaic redox reactions to store the charges at the electrode/electrolyte interface and are generally oxides/ sulfides ( Figure 3.2c). This is Faradic in origin and yields a charge absorption capability of ~2.5 electrons per atom at the accessible surface [10–12].
3 (3) Hybrid ESs, is a combination of the two: electrical double-layer (EDL) and faradaic mechanisms. It is also termed as an asymmetric supercapacitor. While, if one electrode material is a battery type such as PbO2, then the device is a hybrid SC.
SC has a high power density and low energy density. So, various strategies have been adopted by researchers to improve the energy density of the SC cell. Novel cell design (symmetric, asymmetric, hybrid), cell voltage (E ∝ V 2) and synthesizing new electrode nanostructures and electrolyte material opens new doors of opportunity to researchers. Figure 3.3depicts the overview of the different strategies used to improve the energy density of the SC cell [13].
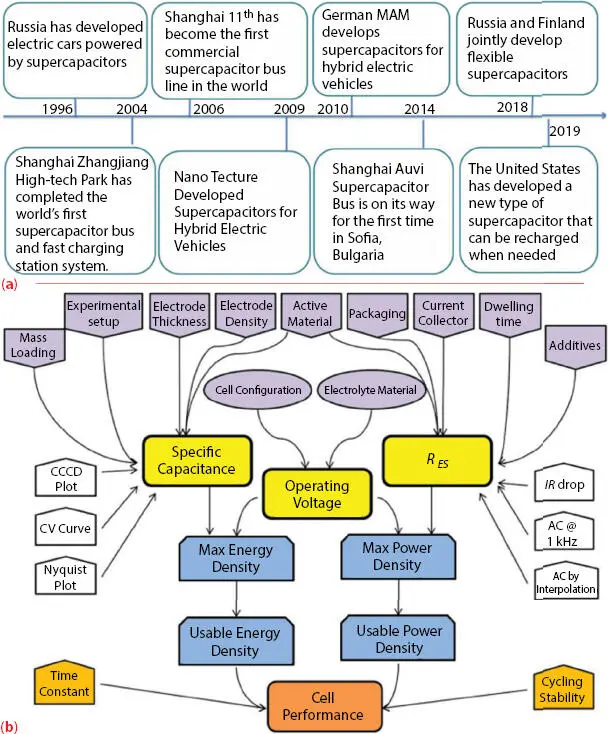
Figure 3.1 (a) The development of supercapacitors in different countries [Reproduced with permission from Ref. [6], © AIP Publishing 2019]. (b) An illustration of key performance metrics, test methods, major affecting factors for the evaluation of SCs [Reproduced with permission from Ref. [7], © Wiley 2014].
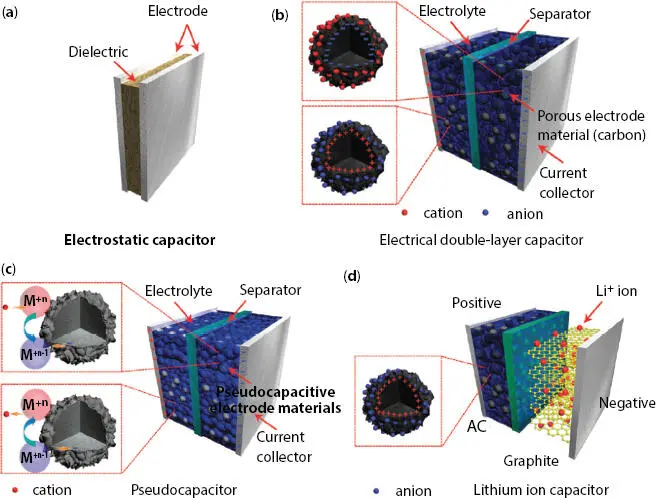
Figure 3.2 Schematic diagram of (a) an electrostatic capacitor, (b) an electric double-layer capacitor, (c) a pseudocapacitor, and (d) a hybrid-capacitor [Reproduced with permission from Ref. [12], © Royal Society of Chemistry 2015].
3.1.2 Key Characteristics of the Electrolyte
In general, the electrolytes for the SC application need to follow some requirements: (1) broad potential window; (2) high ionic conductivity; (3) broad operating temperature range; (4) non-volatile and non-flammable nature; (5) better chemical and electrochemical stability; (6) chemically inert toward SC cell components such as electrodes, current collectors; and (7) cost-effective and environmentally friendly. The prepared polymer electrolyte needs to be examined on the basis of the characteristic parameter that influences the morphological, structural and electrical properties [14–16]. These important parameters are influenced by the host polymer, salt and nanofiller addition. This section discusses the important parameters.
Morphology and Crystallinity
Fast ion dynamics in polymer electrolytes is facilitated by high amorphous content and is examined before going ahead for electrical properties. The X-ray diffraction (XRD) and differential scanning calorimetry (DSC) are techniques to estimate the degree of crystallinity (Χ C ).
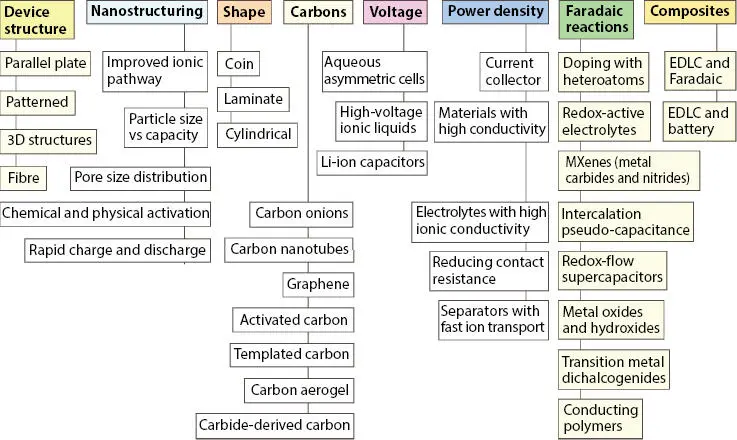
Figure 3.3 Strategies for improving the energy density of supercapacitors [Reproduced with permission from Ref. [13], © Springer Nature 2016].
It provides information about the crystalline and amorphous content in the polymer matrix. From XRD degree of crystallinity is evaluated from the area of crystalline (A C) and amorphous peaks (A A) using expression; 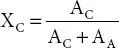 . In DSC the melting enthalpy of crystalline host polymer (ΔH m) and polymer matrix
. In DSC the melting enthalpy of crystalline host polymer (ΔH m) and polymer matrix  are used to evaluate the degree of crystallinity (Χ C) via expression;
are used to evaluate the degree of crystallinity (Χ C) via expression; 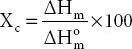 .
.
The ion dynamics in the polymer matrix is examined by evaluating the ionic conductivity and is expressed by relation; 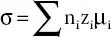 (n iis number of free charge carriers, z i, is ion charge, and & μ iis ion mobility). Ionic conductivity is linked with number of free charge carriers available in the polymer matrix and ion mobility. The ionic conductivity is examined via complex impedance spectroscopy (CIS) technique by applying ac signal (10-100 mV) across the cell assembly SS||PE||SS (SS refers to stainless steel electrode). From the obtained Nyquist plot (Z″ vs. Z′), bulk resistance (R b) is extracted from the intercept on the real axis and ionic conductivity is obtained through this equation;
(n iis number of free charge carriers, z i, is ion charge, and & μ iis ion mobility). Ionic conductivity is linked with number of free charge carriers available in the polymer matrix and ion mobility. The ionic conductivity is examined via complex impedance spectroscopy (CIS) technique by applying ac signal (10-100 mV) across the cell assembly SS||PE||SS (SS refers to stainless steel electrode). From the obtained Nyquist plot (Z″ vs. Z′), bulk resistance (R b) is extracted from the intercept on the real axis and ionic conductivity is obtained through this equation; 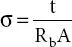 ; where ‘t’ is the thickness of the polymer electrolyte (PE) film, A is the area of the SS electrodes and R bis the bulk resistance.
; where ‘t’ is the thickness of the polymer electrolyte (PE) film, A is the area of the SS electrodes and R bis the bulk resistance.
Читать дальше




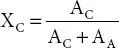 . In DSC the melting enthalpy of crystalline host polymer (ΔH m) and polymer matrix
. In DSC the melting enthalpy of crystalline host polymer (ΔH m) and polymer matrix  are used to evaluate the degree of crystallinity (Χ C) via expression;
are used to evaluate the degree of crystallinity (Χ C) via expression; 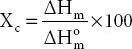 .
.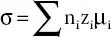 (n iis number of free charge carriers, z i, is ion charge, and & μ iis ion mobility). Ionic conductivity is linked with number of free charge carriers available in the polymer matrix and ion mobility. The ionic conductivity is examined via complex impedance spectroscopy (CIS) technique by applying ac signal (10-100 mV) across the cell assembly SS||PE||SS (SS refers to stainless steel electrode). From the obtained Nyquist plot (Z″ vs. Z′), bulk resistance (R b) is extracted from the intercept on the real axis and ionic conductivity is obtained through this equation;
(n iis number of free charge carriers, z i, is ion charge, and & μ iis ion mobility). Ionic conductivity is linked with number of free charge carriers available in the polymer matrix and ion mobility. The ionic conductivity is examined via complex impedance spectroscopy (CIS) technique by applying ac signal (10-100 mV) across the cell assembly SS||PE||SS (SS refers to stainless steel electrode). From the obtained Nyquist plot (Z″ vs. Z′), bulk resistance (R b) is extracted from the intercept on the real axis and ionic conductivity is obtained through this equation; 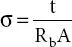 ; where ‘t’ is the thickness of the polymer electrolyte (PE) film, A is the area of the SS electrodes and R bis the bulk resistance.
; where ‘t’ is the thickness of the polymer electrolyte (PE) film, A is the area of the SS electrodes and R bis the bulk resistance.










