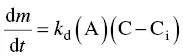On the other hand, if R 1is greater than R c, carbon dioxide diffusion occurs in the opposite direction, and the bubble increases in size, reaching the values R 2, R 3, and R 4. At this last stage, the bubble is subjected to the laws of gravity and starts to rise when its radius reaches the value R 0, leaving behind a new bubble that has started to form. This is how the phenomenon of durable effervescence is achieved.
The fact that the phenomenon of effervescence may be exacerbated due to a large number of microcavities in tartrate microcrystals is an additional reason for ensuring the thorough tartrate stabilization of still wine intended for sparkling wine production. Treatment parameters at this stage must take into account the destabilizing effect of the increase in alcohol content following the second alcoholic fermentation in tank or in bottle.
There are two main types of must and wine treatment technologies for preventing bitartrate instability based on the phenomenon of low‐temperature crystallization (Section 12.3.2). The first uses traditional slow stabilization technology ( Section 1.7.2), as opposed to the more recent Müller‐Späth rapid contact stabilization process (1979), where the wine is seeded with cream of tartar crystals ( Section 1.7.3). There are two variants of the rapid process, one static and the other dynamic, known as “continuous treatment.”
Besides these two systems, a new separation technique, electrodialysis, is also applied to the bitartrate stabilization of wine (Section 12.5.3). The use of ion‐exchange resins is also permitted in certain countries, including the United States (Section 12.4.3). Finally, it is possible to prevent the precipitation of these salts by adding crystallization inhibitors, such as metatartaric acid ( Section 1.7.6), yeast mannoprotein extracts ( Section 1.7.7), or carboxymethylcellulose (CMC) ( Section 1.7.8).
1.5.2 Tartrate Crystallization and Precipitation
The two artificial cold stabilization technologies described elsewhere ( Sections 1.7.2– 1.7.4) do not use the same crystallization mechanism. The traditional stabilization process involves spontaneous, primary nucleation, a slow process that produces large crystals because the nuclei grow slowly. In rapid stabilization processes, the awkward stage of primary nucleation is replaced by a fast, homogeneous secondary nucleation. This is induced by adding massive quantities of small exogenous tartrate crystals, which also considerably boost supersaturation (C − S, difference between the real concentration and the concentration at saturation).
Furthermore, in this technique, the temperature of the wine is reduced abruptly, promoting the formation of small endogenous tartrate nuclei, i.e. significantly increasing the surface area (A) of the liquid–solid interface by maximizing the diffusion of bitartrate aggregates with pre‐crystalline structures, thus ensuring faster growth of the nuclei ( Figure 1.13).
It has been experimentally verified (Maujean et al ., 1986) that the crystallization rate, monitored by measuring the electrical conductivity of wine, is directly proportional to the surface area of the liquid–solid interface represented by the nuclei. This result is consistent with the following equation, proposed by Dunsford and Boulton (1981), defining the mass velocity at which the pre‐crystalline aggregates of potassium bitartrate diffuse toward the surface (A) of the adsorption interface:
(1.9) 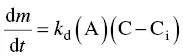
where C is the concentration of the solution and C iis the concentration at the interface.
One practical application of these theoretical results is that producers and distributors have been obliged to ensure that their cream of tartar particles have a radius of less than 40 μm. This parameter is also important as nuclei with a radius greater than 200 μm grow much more slowly than smaller nuclei.
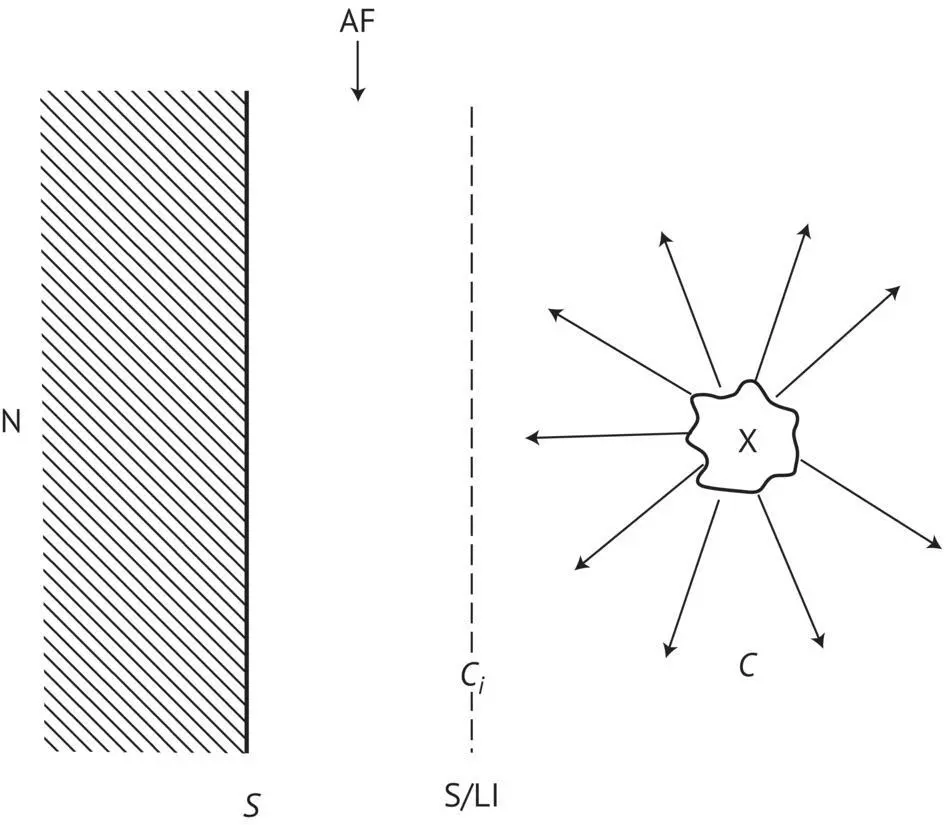
FIGURE 1.13 Diagram illustrating the importance of the diffusion speed of KHT aggregates toward the solid–liquid adsorption interface for the growth of nuclei: AF, adsorption film; X, molecular aggregate of KHT diffusing toward the interface; S/LI, solid–liquid interface; N, nuclei; C , KHT concentration in the liquid phase; C i, KHT concentration at the solid–liquid interface; S, theoretical solubility of KHT; C − S, supersaturation state of the wine; C > C i > S.
This confirms the findings of Devraine (1969), who also concluded that large nuclei stop growing as they release “fines,” i.e. daughter nuclei. This observation explains the continued effectiveness in stabilizing white wines of cream of tartar that has been recycled five times, provided that the particles were initially very small. In contrast, it is not possible to recycle cream of tartar so many times in red wines due to the affinity between tartaric acid and phenols, known to be powerful crystallization inhibitors.
Another advantage of the contact process is that seeding with small cream of tartar particles enhances the state of supersaturation (C − C i). This is important as the crystallization rate is proportional not only to the interface value (A) but also to the state of supersaturation (C − C i) ( Equation (1.9)).
The added cream of tartar must be maintained in suspension homogeneously, throughout the tank by appropriate agitation, so that the nuclei provide a maximum contact interface with the aggregates of endogenous tartrate. As soon as the cream of tartar is added, the crystallization rate depends solely on the interface factor (A), as (C − C i) is so large that it may, at least in the first hour of contact, be considered constant.
It may therefore be stated that, during the first hour, the crystallization rate depends solely on the rate of diffusion of the aggregates. After this initial contact time, the nuclei have grown but, more importantly, (C − C i) has decreased, as the very high crystallization rate has consumed large quantities of exogenous tartrate. In other words, A, i.e. the diffusion rate, is no longer the limiting factor, but rather the state of supersaturation (C − C i). As C tends toward C i, the situation in the wine approaches the theoretical solubility (S) of tartrate under these treatment conditions. Therefore, by the end of the treatment process, the crystallization rate is controlled more by thermodynamics than kinetics.
These theoretical considerations, applied to a short treatment involving seeding with tartrate crystals, show that great care and strict supervision are required to ensure the effectiveness of artificial cold stabilization. The following factors need to be closely monitored: the wine's initial state of supersaturation, the particle size of the added tartrates, the seeding rate, the effectiveness of agitation at maintaining the crystals in suspension, treatment temperature, and, finally, contact time.
1.5.3 Using Electrical Conductivity to Monitor Tartrate Precipitation
Wurdig and Muller (1980) were the first to make use of the capacity of must and wine to act as electrolytes, i.e. solutions conducting electricity, to monitor tartrate precipitation. Indeed, during precipitation, potassium bitartrate passes from the dissolved, ionized state, when it is an electrical conductor, to a crystalline state, when it precipitates and is no longer involved in electrical conductivity:
Читать дальше