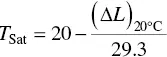TABLE 1.13Values of the Concentration Products of Wines and the Corresponding Percentage Drop in Conductivity Produced by the Mini‐Contact Test
| Samples |
CP K× 10 5 |
Drop in conductivity at 0°C (%) |
| A |
7.28 |
0.5 |
| B |
11.62 |
1.0 |
| C |
11.84 |
0.0 |
| D |
12.96 |
1.5 |
Table 1.15shows that the effects of variations in cream of tartar particle size and contact time in the same wine are capable of causing a 5% difference in the drop in initial conductivity, which is the benchmark for deciding whether a wine is stable or not.
In practice, a rapid response test is required for monitoring the effectiveness of artificial cold stabilization. The preceding results show quite clearly that the tests based on induced crystallization are relatively unreliable for predicting the stability of a wine at 0°C.
1.6.3 The Wurdig Test and the Concept of Saturation Temperature in Wine
Wurdig et al . (1982) started with the idea that the more KHT a wine is capable of dissolving at low temperatures, the less supersaturated it is with this salt and, therefore, the more stable it should be in terms of bitartrate precipitation. The authors defined the concept of saturation temperature ( T Sat) in a wine on the basis of this approach.
The saturation temperature of a wine is the lowest temperature at which it is capable of dissolving potassium bitartrate. In this test, temperature is used as a means of estimating the bitartrate stability of a wine, on the basis of the solubilization of a salt.
In comparison with the previously described tests, based on crystallization, this feature seems very convincing. Indeed, the solubilization of a salt is a spontaneous, fast, repeatable phenomenon, much less dependent on the particle size of the added tartrate crystals. The solubilization of KHT is also much less affected by the colloidal state of the wine at the time of testing. It has been observed that protective colloids act as crystallization inhibitors, but do not affect the solubilization of salts. Consequently, estimating the bitartrate stability of a wine by testing the solubilization of KHT, i.e. saturation temperature, is a more reliable measurement in the long term as it is independent of any colloidal reorganization during storage and aging.
TABLE 1.14Limitations of the Reliability of the Mini‐Contact Test in Assessing the Stability of a Wine by Adding Increasing Quantities of Potassium Bitartrate and Measuring the Percentage Drop in Conductivity
| Samples |
pH |
K +(mg/l) |
CP K× 10 5 |
Drop in initial conductivity (%) |
| Control |
3 |
390 |
9.17 |
1.5 |
| Wine + 0.2 g/l KHT |
3 |
420 |
10.85 |
11.5 |
| Wine + 0.5 g/l KHT |
3.03 |
469 |
13.33 |
7.5 |
| Wine + 0.7 g/l KHT |
3.05 |
513 |
15.26 |
12.5 |
| Wine + 1 g/l KHT |
3.06 |
637 |
21.16 |
11.5 |
TABLE 1.15Influence of Tartrate Particle Size and Mini‐Contact Test Time on the Percentage Drop in Conductivity of the Wine
| Drop in conductivity (%) |
Commercial KHT |
KHT: particle size greater than 100 μm |
KHT: particle size smaller than 63 μm |
| After 10 min |
12 |
9 |
14 |
| After 20 min |
13 |
11 |
16 |
The saturation temperature of a wine was determined by measuring electrical conductivity ( Figure 1.14) in a two‐stage experiment.
In the first experiment, the wine is brought to a temperature of approximately 0°C in a temperature‐controlled bath equipped with sources of heat and cold. The temperature is then raised to 20°C in 0.5°C increments, and the wine's conductivity measured after each temperature change. In this way, it can be observed that the variation in conductivity according to the temperature of a wine containing no KHT crystals is represented by a roughly straight line.
In the second experiment, a volume (100 ml) of the same wine is brought to a temperature close to 0°C, 4 g/l of KHT crystals is added, and the temperature is once again raised to 20°C in 0.5°C increments. The wine is agitated constantly, and its conductivity measured after each temperature change. Two patterns are observed:
1 Subsequent to the addition of 4 g/l of KHT, the wine ( Figure 1.14a) shows a linear variation in conductivity at low temperatures that could almost be superimposed on that of the wine without crystals, until a temperature TSat, where the conductivity leaves the straight line and follows the exponential solubility curve. FIGURE 1.14 Experimental determination of the saturation temperature of a wine by the temperature gradient method (Wurdig et al., 1982). (a) Example of a wine that is not highly supersaturated, in which no induced crystallization occurs after the addition of potassium bitartrate crystals at low temperature. (b) Example of a highly supersaturated wine, in which induced crystallization occurs immediately after the addition of potassium bitartrate crystals.
2 Following the addition of 4 g/l of KHT, the wine's conductivity ( Figure 1.14b) at temperatures around 0°C is below that of the wine alone. This means that low‐temperature induced crystallization has occurred, revealing a state of supersaturation with high endogenous KHT levels in the wine. Its conductivity then increases in a linear manner until temperature TA; then the KHT starts to dissolve, and the conductivity follows the exponential solubility curve. At temperature TB, the exponential solubility curve crosses the straight line showing the conductivity of the wine alone. This intersection corresponds to the wine's true saturation temperature. The temperature TA corresponds to that of the same wine after “contact,” leading to desaturation caused by induced crystallization. It is therefore normal that, following desaturation, the wine should solubilize more KHT, at a temperature lower than its true saturation temperature, TB.
On a production scale, where rapid stabilization technologies are used, experimental determination of the saturation temperature by the temperature gradient method is incompatible with the rapid response required to monitor the effectiveness of ongoing treatment.
On the basis of statistical studies of several hundred wines, Wurdig et al . (1982) established a linear correlation defined by
(1.10) 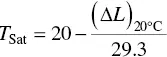
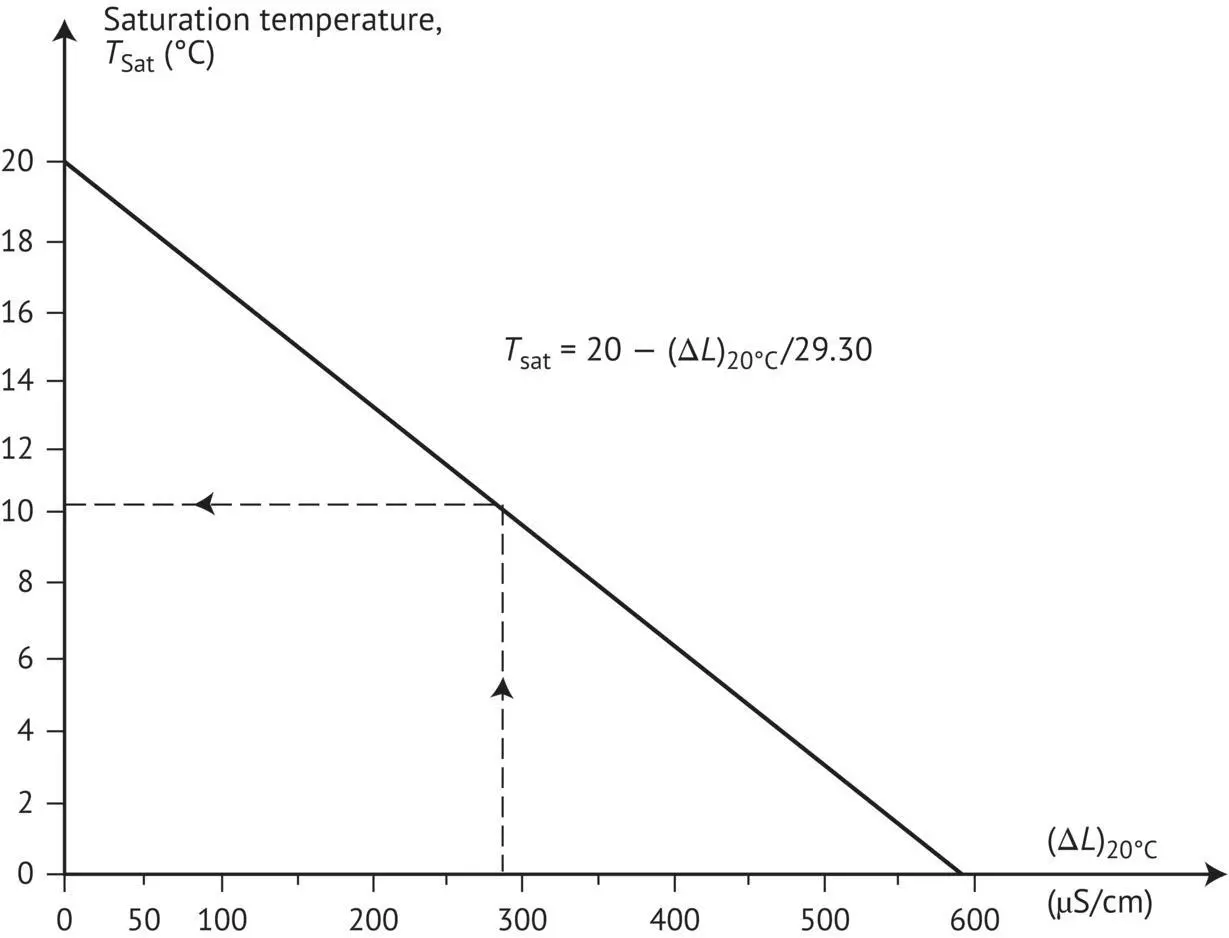
FIGURE 1.15 Determining the saturation temperature of a wine according to the variation (Δ L ) in conductivity at 20°C before and after the addition of potassium bitartrate (KHT) (Wurdig et al ., 1982).
This straight‐line correlation ( Figure 1.15) between the variation in conductivity of a wine at 20°C before and after the addition of 4 g/l of cream of tartar (Δ L ) and the saturation temperature has only been verified for wines where the solubilization temperature of KHT is between 7 and 20°C. The practical advantage of using this equation is that the saturation temperature of a wine may be determined in just a few minutes, using only two measurements.
In some wines, crystallization may be induced by adding cream of tartar at 20°C. This means that they have a lower conductivity after the addition of tartar, i.e. a saturation temperature above 20°C. This is most common in rosé and red wines. To determine their precise saturation temperature, the samples are heated to 30°C. Cream of tartar is added, and the increase in conductivity at this temperature is measured. The saturation temperature is deduced from (Maujean et al ., 1985)
Читать дальше