While potassium bitartrate is perfectly soluble in water, it is relatively insoluble in alcohol. Thus, in a dilute alcohol solution at 10% v/v and 20°C, its solubility (S) is only 2.9 g/l.
The potassium concentration in wine is frequently as high as 780 mg/l or 20 mEq/l, i.e. 3.76 g/l of potassium bitartrate. Therefore, the concentration (C) of the salt is greater than its solubility (S). It follows that the product CP of the real concentrations (r):
(1.6) 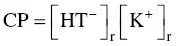
is greater than the solubility product (SP) defined by:
(1.7) 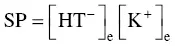
according to the solubility equilibrium:
(1.8) 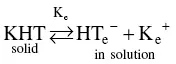
In this equation, the concentrations (e) of HT −anions and K +cations are theoretically obtained at the thermodynamic equilibrium of the solid KHT/dissolved KHT system, under the temperature and pressure conditions in wine.
The diagram ( Figure 1.11) presenting the states of potassium bitartrate in a system correlating the temperature/concentration axes with conductivity shows three states: 1, 2, and 3, with borders defined by the exponential solubility (A) and supersolubility (B) curves. The exponential solubility curve (A) is obtained by adding 4 g/l of crystallized KHT to a wine. The increase in the wine's electrical conductivity according to temperature is then recorded. This corresponds to the dissolving and ionization of tartrates. As explained in Section 1.6.4, conductivity values correspond to saturation temperatures ( T Sat), since wine is capable of dissolving increasing amounts of KHT as the temperature rises. The exponential solubility curve represents the boundary between two possible states of KHT in a wine according to temperature. Thus, at a constant concentration (or conductivity), when the temperature of the wine rises, KHT changes from state 2, where it is supersaturated and supercooled, to state 1, i.e. dissolved, where its concentration product (CP) is lower than its SP.
The exponential supersolubility curve (B) is obtained experimentally and geometrically from the envelope linking the spontaneous crystallization temperature (TCS i) points of a wine brought to various states of supersaturation by completely dissolving added KHT and then reducing the temperature of the wine until crystallization is observed. The exponential supersolubility curve represents the boundary between state 2, where potassium bitartrate is in a state of supersaturation (C − S ) and supercooling, and state 3, where it is crystallized.
Once the exponential solubility (A) and supersolubility (B) curves have been defined, it is possible to determine the state of a wine at a known temperature with considerable accuracy. Indeed, any wine with a KHT concentration, or conductivity, above that defined by the intersection of the vertical line drawn upward from the temperature of the wine and the exponential solubility curve (A) is in a supersaturated state, and so, theoretically, there is a probability of spontaneous crystallization. The crystallization phenomenon will, in fact, be observed at the intersection of the same vertical line and the exponential supersolubility curve (B). It appears, therefore, that supersaturation is necessary, but not sufficient, for primary nucleation phenomena and spontaneous crystallization to occur in a wine.
The delay in crystallization of a salt in relation to its solubilization, which is partially responsible for the supersaturated state in supercooled form, is due to lack of energy.
The formation of a small crystal, known as a nucleus, in a liquid phase corresponds to the creation of an interface between two phases. This requires a great deal of energy, known as interfacial surface energy. In a wine, the width DS of the domain of supersaturation ( Figure 1.11), expressed in degrees Celsius, is increased by the presence of macromolecules that inhibit the growth of nuclei and crystallization of KHT. These macromolecules, known as “protective colloids,” include proteins and condensed tannins, and also carbohydrate polymers, such as pectins and gums, i.e. neutral polysaccharides. Besides these chemical macromolecules, there are also more complex polymers, such as glycoproteins, e.g. mannoproteins of yeast origin (Lubbers et al ., 1993).
The impact of the protective colloid effect on the bitartrate stabilization of a wine varies according to the winemaking methods used. Red wines have a higher phenol content than white wines, and their condensed tannins have a strong inhibiting effect.
In its natural state, wine is always supersaturated and therefore unstable. This situation may be more or less durable, depending on the reorganization of the colloids that occurs during aging. Storage temperatures may be decisive in triggering bitartrate crystallization.
It is certainly true that spontaneous crystallization, under natural conditions, is an unreliable, unpredictable phenomenon. This is why the production process for many red and white wines includes artificial cold stabilization before bottling. This type of treatment is justified, especially as consumers will not tolerate the presence of crystals, even if they do not affect quality.

FIGURE 1.11 Determination of the exponential solubility (A) and supersolubility (B) curves of potassium bitartrate in a wine. Evaluation of the supersaturation and instability domains according to KHT content (Maujean et al ., 1985). DS, domain of supersaturation; 1, dissolved KHT; 2, supersaturated, supercooled KHT; 3, crystallized KHT;  , spontaneous crystallization temperature when 1.1 g/l KHT is added;
, spontaneous crystallization temperature when 1.1 g/l KHT is added; 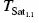 , saturation temperature of a wine in which 1.1 g/l KHT has been dissolved.
, saturation temperature of a wine in which 1.1 g/l KHT has been dissolved.
Furthermore, artificial cold stabilization is indispensable for sparkling wines. Indeed, microcavities in the surface of the glass or in solid particles in suspension, especially microcrystals of potassium bitartrate, may lead to the formation of too many bubbles when the bottle is opened, causing excessive effervescence known as “spraying.” This is sometimes responsible for the loss of large quantities of wine during disgorging, or when bottles are opened by consumers (Volume 1, Section 14.3.4). The origin of this effervescence and spraying is given by the repetitive bubble formation model (Casey, 1988; Figure 1.12). This bubble degassing model is based on the phenomenon of heterogeneous induced nucleation.
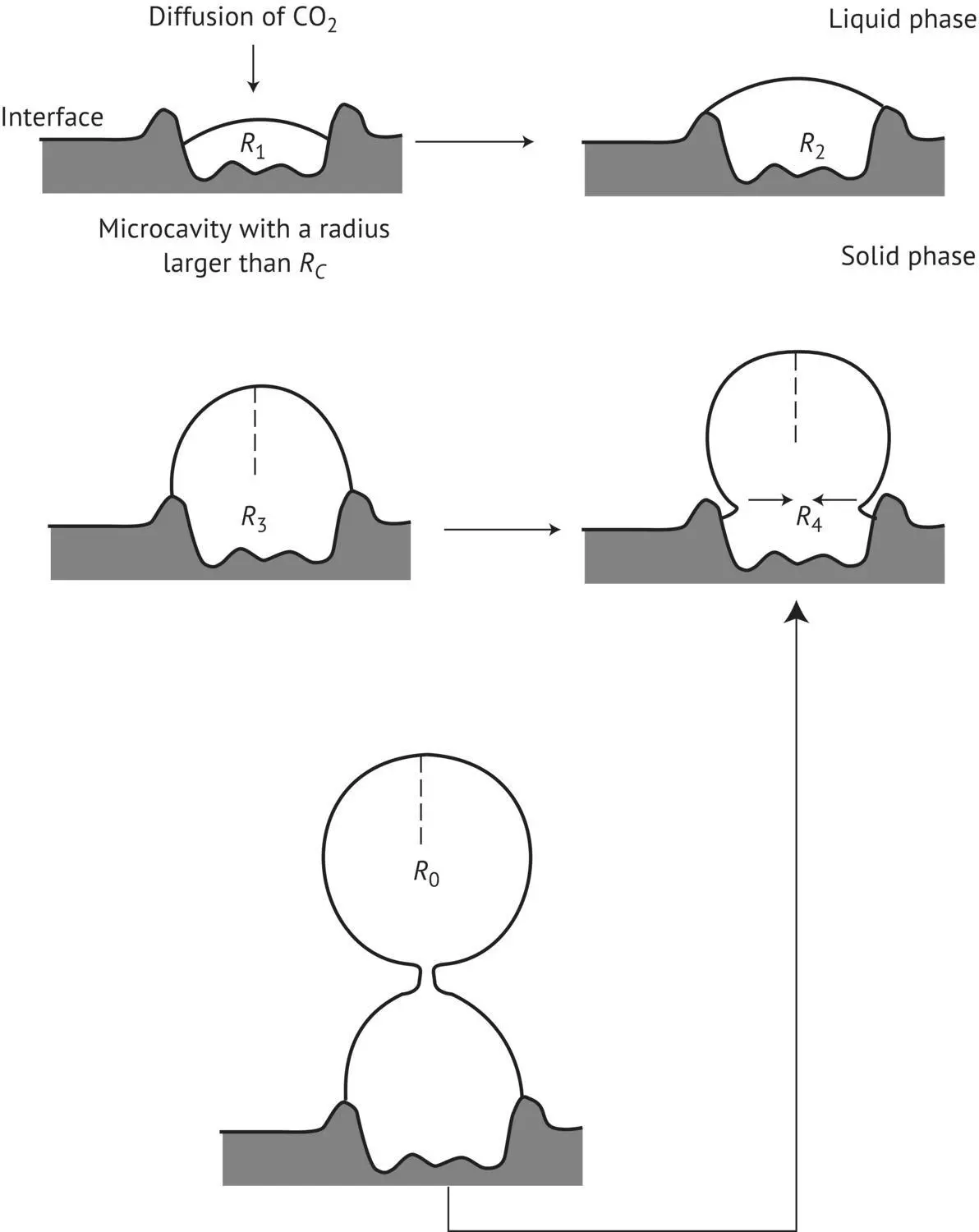
FIGURE 1.12 Repetitive bubble formation on a microcavity in a tartrate microcrystal in a sparkling wine. Heterogeneous induced nucleation, according to the Casey model (1988).
However, nucleation may be induced, and the microcavities are efficient only if they have a radius R 1greater than a critical radius R cdefined by Laplace's law. Indeed, below this value, the excess pressure in the bubble is such that carbon dioxide passes from the gas phase to the liquid phase and so the bubble disappears.
Читать дальше

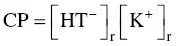
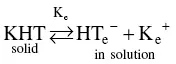

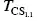 , spontaneous crystallization temperature when 1.1 g/l KHT is added;
, spontaneous crystallization temperature when 1.1 g/l KHT is added; 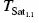 , saturation temperature of a wine in which 1.1 g/l KHT has been dissolved.
, saturation temperature of a wine in which 1.1 g/l KHT has been dissolved.











