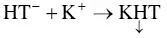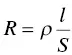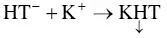
The principle of measuring conductivity consists in making the wine into an electrical conductor, defined geometrically by the distance l separating two platinum electrodes with S ‐shaped cross sections. The resistance R (in ohms) of the conductor is defined by the relation:
TABLE 1.12Resistivity and Conductivity of a KCl (0.02 M) Solution According to Temperature (in °C).
| Temperature (°C) |
15 |
16 |
17 |
18 |
19 |
20 |
21 |
22 |
23 |
24 |
25 |
| Resistivity (Ω/cm) |
446 |
436 |
426 |
417 |
408 |
400 |
392 |
384 |
376 |
369 |
362 |
| Conductivity (μS/cm) |
2,242 |
2,293 |
2,347 |
2,398 |
2,451 |
2,500 |
2,551 |
2,604 |
2,659 |
2,710 |
2,769 |
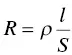
In this equation, ρ is the resistivity. Its inverse ( γ ) is the conductivity expressed in siemens per meter (S/m) or microsiemens per centimeter (μS/cm = 10 −4S/m).
The expression of resistivity ρ = RS / l involves the term S / l , known as the cell k constant. This constant is specific to each cell, according to its geometry, and may also vary with use, due to gradual deterioration of the electrodes or the effect of small impacts.
It is therefore necessary to check this constant regularly and to determine it at a conductivity close to that of wine. In practice, a 0.02 M KCl solution is used. The temperature of the KCl (0.02 M) solution must be taken into account when checking the cell constant. The resistivity and conductivity values of this solution according to temperature are specified in Table 1.12.
The conductivity meter cell is subjected to an alternating current. The frequency is set at 1 kHz for the standardized solution (KCl = 0.02 M) and for wine to avoid polarizing the electrodes. A conductivity meter is used for continuous monitoring of tartrate precipitation in wine (see Section 1.6.4, Figure 1.16).
1.6 Tests for Predicting Wine Stability
1.6.1 The Refrigerator Test
This traditional test is somewhat empirical. A sample (approximately 100 ml) of wine, taken before or after artificial cold stabilization, is stored in a refrigerator for four to six days at 0°C and then inspected for crystals. In the case of wines intended for a second fermentation, alcohol may be added to increase the alcohol content by 1.3–1.5% vol. This simulates the effects of the second fermentation and makes it possible to assess the bitartrate stability of the finished sparkling wine.
The advantages of this test are that it is simple and practical and requires no special equipment. On the other hand, it is mainly qualitative and does not provide an accurate indication of the wine's degree of instability. Its major disadvantage is that it takes a long time and is incompatible with short contact stabilization technologies, where rapid results are essential to assess the treatment's effectiveness in real time.
Finally, this test is neither reliable nor easily repeatable, as it is based on the phenomenon of spontaneous, non‐induced crystallization—a slow, undependable process.
1.6.2 The Mini‐Contact Test
A sample of wine with 4 g/l added potassium bitartrate is maintained at a temperature of 0°C for two hours and constantly agitated. The wine sample is cold filtered, and the weight increase of the tartrate collected (exogenous tartrate + wine tartrate) is assessed. It is also possible to dissolve the precipitate in a known volume of hot water and measure the increase in acidity as compared with that of the 4 g/l exogenous potassium bitartrate added to the wine.
The mini‐contact test is based on homogeneous induced nucleation, which is faster than primary nucleation. However, this test does not take into account the particle size of the seed tartrate, although the importance of its effect on the crystallization rate is well known. The operative factor in this test is the surface area of the liquid–solid contact interface. Furthermore, this test defines the stability of the wine at 0°C and in its colloidal state at the time of testing. In other words, it makes no allowance for colloidal reorganization in wine, especially red wine, during aging.
It is normal to find potassium bitartrate crystals, associated with precipitated condensed coloring matter, in wine with several years' aging. When phenols condense, they become bulky, precipitate, and are no longer able to express their protective colloid effect.
It should be noted that mini‐contact test results tend to overestimate a wine's stability and therefore the effectiveness of prior treatment. This statement is based on work by Boulton (1982). After two hours' contact, only 60–70% of the endogenous tartrate has crystallized, and therefore the increase in weight of the crystal precipitate is lower than it could be. These results are interpreted to mean that the treatment was more effective, or the wine more stable, than was actually the case. To make the mini‐contact test faster, more reliable, and compatible with the dynamic contact process, the Martin Vialatte company proposed the following variant in 1984: seeding a wine sample with 10 g/l of cream of tartar and measuring the drop in conductivity at 0°C.
The rules governing stability under the extreme supersaturation conditions prevailing in wine are as follows:
1 If, in the 5–10 minutes after seeding, the drop in conductivity is no more than 5% of the wine's initial conductivity (measured before adding potassium bitartrate), the wine may be considered to be properly treated and stabilized.
2 If the drop in conductivity is over 5%, the wine is considered unstable.
As this test is based on measuring the wine's electrical conductivity, it has the tremendous advantage that there is no need to collect the precipitate by filtration and determine the increase in weight. This new mini‐contact test, measuring conductivity, is much faster (5–10 minutes instead of two hours). Furthermore, by comparison with the first variant of the mini‐contact test, as the contact surface (A) and, consequently, the state of supersaturation of the wine are multiplied by 2.5 (adding 10 g/l of KHT instead of 4 g/l), it gives a more accurate assessment of a wine's stability.
In spite of these improvements, this test remains open to criticism, and its reliability is limited. Indeed, as is the case with the preceding test, it does not always take into consideration the effect of particle size and is based on excessively small variations in conductivity and too short a contact time. The results in Tables 1.13– 1.15corroborate this point of view. In Table 1.13, results indicate that a variation of over five units in the concentration product CP K(see samples A and D) only caused a 1% decrease from the wine's initial conductivity. In this instance, a white wine with a CP Kclose to 13 was considered unstable, but this assessment was not confirmed by the percentage drop in conductivity.
The unreliability of this result is confirmed by the experiment described in Table 1.14, involving a wine with an initial CP Kof 9.17 × 10 5, maintained at 30°C, in which increasing concentrations of commercial cream of tartar were dissolved. It was observed that when the CP Kof a wine was doubled (e.g. between wine +0.2 g/l of dissolved KHT and wine +1 g/l of dissolved KHT) the percentage drop in conductivity was the same, although there was obviously a difference in stability.
Читать дальше