On the other hand, it is a good thing that wines have such low pH values, as this enhances their microbiological and physicochemical stability. Low pH hinders the development of microorganisms while increasing the antiseptic fraction of sulfur dioxide. The influence of pH on physicochemical stability is due to its effect on the solubility of tartrates, in particular potassium bitartrate but, above all, calcium tartrate and the double salt calcium tartromalate.
Iron casse, or iron haze, is also affected by pH. Indeed, iron (III), or ferric iron, establishes soluble complexes with molecules such as citric acid. These complexes are destabilized by increasing pH to produce insoluble salts, such as ferric phosphates (see “white casse”) or even ferric hydroxide, Fe(OH) 3.
1.4.2 Expression of pH in Wine
Wines are mixtures of weak acids, combined to form salts to a greater or lesser extent according to their p K a( Table 1.3). The proportion of salts also depends on geographical origin, grape variety, the way the vines are trained, and the pressing and winemaking methods used.
Due to their composition, musts and wines are acid–base “buffer” solutions, i.e. a modification in their chemical composition produces only a limited variation in pH. This explains the relatively small variations in the pH of must during alcoholic and malolactic fermentations.
The pH of a solution containing a weak monoprotic acid and its strong basic salt proves the Henderson–Hasselbalch equation:
(1.2) 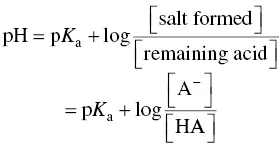
This equation is applicable to must and wine, where the most important acids are diacids. It is an approximation, assuming the additivity of the acidity contributed by each acid to the total. The application of Equation (1.2)also makes the simplifying assumption that the degree to which the acids are combined in salts is independent of each other. These assumptions are currently being challenged. Indeed, recent research has shown that organic acids react among themselves, as well as with amino acids (Dartiguenave et al ., 2000a).
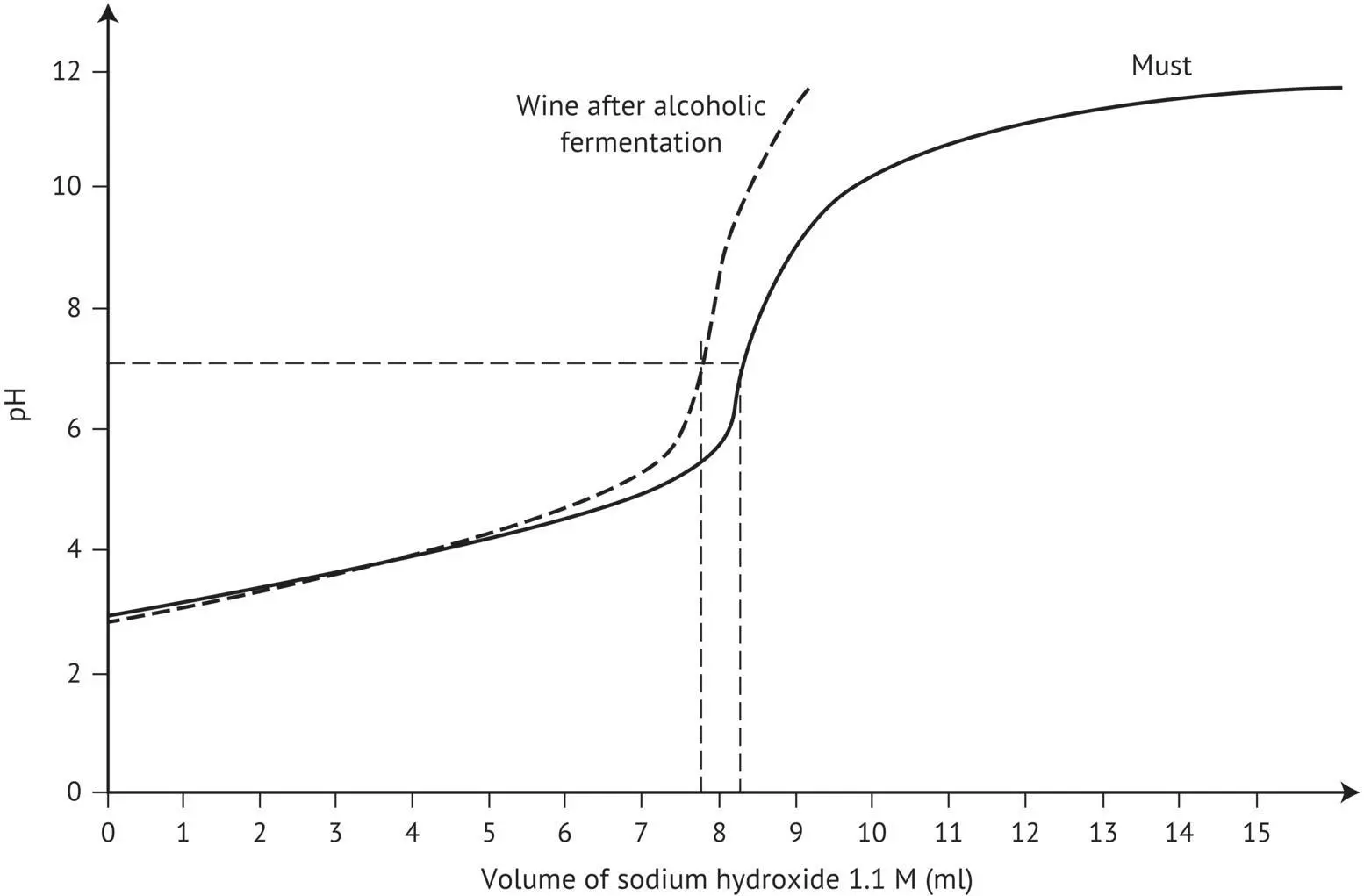
FIGURE 1.3 Comparison of the titration curves of a must and the corresponding wine.
Comparison ( Table 1.3) of the p K avalues of tartaric (3.01), malic (3.46), lactic (3.81), and succinic (4.18) acids leads to the conclusion that tartaric acid is the “strongest,” so it will take priority in forming salts, displacing, at least partially, the weaker acids. In reality, all of the acids interact. Experimental proof of this is given by the neutralization curve of a must, or the corresponding wine, obtained using sodium or potassium hydroxide ( Figure 1.3). These curves have no inflection points at pH corresponding to the p K avalues of the various acids, as there is at least partial overlapping of the maximum “buffer” zones (p K a± 1). Thus, the neutralization curves are quasi‐linear for pH values ranging from 10 to 90% neutralized acidity. They therefore indicate a constant buffer capacity in this zone. From a more quantitative standpoint, a comparison of the neutralization curves of must and the corresponding wine shows that the total acidity values, assessed by the volume of sodium hydroxide added to obtain pH 7, differ by 0.55 mEq. In the example described above, both must and wine samples were 50 ml, and the total acidity of the wine was 11 mEq/l (0.54 g/l as H 2SO 4) lower than that of the must. This drop in total acidity in wine may be attributed to a slight consumption of malic acid by the yeast during alcoholic fermentation, as well as a partial precipitation of potassium bitartrate (insoluble in alcohol).
The slope of the linear segment of the two neutralization curves differs noticeably. The curve corresponding to the must has a gentler slope, showing that it has a greater buffer capacity than the wine.
The next paragraph gives an in‐depth description of this important physicochemical parameter of wine.
1.4.3 The “Buffer” Capacity of Musts and Wines
The acid–base buffer capacity of wines is largely responsible for their physicochemical and microbiological stability, as well as their flavor balance. For example, the length of time a wine leaves a fresh impression on the palate is directly related to the formation of acid salts by basic proteins in saliva, i.e. the expression of the buffer phenomenon and its capacity. In contrast a wine that tastes “flat” has a low buffer capacity, but this does not necessarily mean that it has a low acidity level. At a given total acidity level, buffer capacity varies according to the composition and type of acids present. This point will be developed later in this chapter.
In a particular year, a must's total acidity and acid composition depend mainly on geography, soil conditions, and climate, including soil humidity and permeability, as well as rainfall patterns, and, above all, temperature. Temperature determines the respiration rate, i.e. the combustion of tartaric and, especially, malic acids in grape flesh cells. The predominance of malic acid in must from cool‐climate vineyards is directly related to temperature, while malic acid is eliminated from grapes in hotter regions by combustion and is thus found in much lower amounts than tartaric acid.
Independently of climate, grape growers and winemakers have some control over total acidity and even the acid composition of the grape juice during ripening. Leaf thinning and shoot trimming restrict acid biosynthesis and, above all, combustion by reducing the greenhouse effect of the leaf canopy. Another way of controlling total acidity levels is by choosing the harvesting date. Grapes intended for sparkling wines must be picked at the correct level of technological ripeness to produce must with a total acidity of 9–10 g/l as H 2SO 4. This acidity level is necessary to maintain the wines' freshness and, especially, to minimize color leaching from the red grape varieties, Pinot Noir and Pinot Meunier, used in Champagne. At this stage in the ripening process, the grape skins are much less fragile than they are when completely ripe. The last method for controlling the total acidity of must is by taking great care in pressing the grapes and keeping the juice from each pressing separate (Volume 1, Section 14.3.2). In the Champagne region, the cuvée corresponds to juice from the mid‐part of the flesh (furthest from the skin and seeds), where it has the highest sugar and acidity levels.
Once the grapes have been pressed, winemakers have other means of raising or lowering the acidity of a must or wine. It may be necessary to acidify “flat” white wines by adding tartaric acid after malolactic fermentation in years when the grapes have a high malic acid content. This is mainly the case in cool‐climate vineyards, where the malic acid is not consumed during ripening. The disadvantage is that it causes an imbalance in the remaining total acidity, which then consists exclusively of a diacid, tartaric acid, and its monopotassium salt.
One method that is little‐known, or at least rarely used to avoid this total acidity imbalance, consists in partially or completely eliminating the malic acid by chemical means using a mixture of calcium tartrate and calcium carbonate. This method precipitates the double salt, calcium tartromalate ( Section 1.4.4, Figure 1.9), and is a very flexible process. When the malic acid is partially eliminated, the wine has a buffer capacity based on those of both tartaric and malic acids, and not just on that of the former. Tartrate buffer capacity is less stable over time, as it decreases due to the precipitation of monopotassium and calcium salts during aging, whereas the malic acid salts are much more soluble.
Читать дальше














