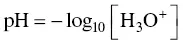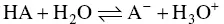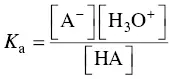Even using the latest techniques, it is difficult to predict the total acidity of a wine on the basis of the acidity of the must from which it is made, for a number of reasons.
Part of the original fruit acids may be consumed by yeasts and, especially, bacteria (see malolactic fermentation). On the other hand, yeasts and bacteria produce acids, e.g. succinic and lactic acids. Furthermore, acid salts become less soluble as a result of the increase in alcohol content. This is the case, in particular, of the monopotassium form of tartaric acid, which causes a decrease in total acidity on crystallization, as potassium bitartrate (potassium hydrogen tartrate [KHT]) still has a carboxylic acid function.
In calculating total acidity, a correction should be made to allow for the acidity contributed by sulfur dioxide and carbon dioxide. Sulfuric acid is much stronger 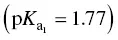 than carbonic acid
than carbonic acid 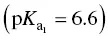 .
.
In fact, high concentrations of carbon dioxide tend to lead to overestimation of total acidity, especially in slightly effervescent (spritzy) wines and even more so in sparkling wines. This is also true of young wines, which always have a high CO 2content just after fermentation.
Wines must therefore be degassed prior to analyses of both total and volatile acidity.
Volatile acidity in wine is a highly important physicochemical parameter to be monitored by analysis throughout the winemaking process. Although it is an integral part of total acidity, volatile acidity is clearly considered separately, even if it only represents a small fraction in quantitative terms.
This value has always been, quite justifiably, linked to wine quality. Indeed, when an enologist tastes a wine and decides there is excessive volatile acidity, this unfavorable assessment has a negative effect on the wine's value. This organoleptic characteristic is related to an abnormally high concentration of acetic acid, in particular, as well as a few homologous carboxylic acids. These compounds are distilled when wine is evaporated. Those which, on the contrary, remain in the residue constitute fixed acidity.
Volatile acidity in wine consists of free and salt forms of volatile acids. This explains why the official assay method for volatile acidity, by steam distillation, requires the salt fractions to be rendered free and volatile by acidifying the wine with tartaric acid (approximately 0.5 g per 20 ml). Tartaric acid is stronger than the volatile acids, so it displaces them from their salts.
In France, both total and volatile acidity are usually expressed in grams per liter of sulfuric acid. An appellation d'origine contrôlée (controlled designation of origin) wine is said to be “of commercial quality” if volatile acidity does not exceed 0.9 g/l of H 2SO 4, i.e. 1.35 g/l expressed as tartaric acid and 1.1 g/l as acetic acid. Acetic acid, the principal component of volatile acidity, is mainly formed during fermentation.
Alcoholic fermentation of grapes normally leads to the formation of 0.2–0.3 g/l (as H 2SO 4) of volatile acidity in the corresponding wine. The presence of oxygen always promotes the formation of acetic acid. Thus, this acid is formed both at the beginning of alcoholic fermentation and toward the end when the process slows down. In the same way, an increase in volatile acidity of 0.1–0.2 g/l (as H 2SO 4) is observed during malolactic fermentation. Work by Chauvet and Brechot (1982) established that acetic acid is formed during malolactic fermentation due to the breakdown of citric acid by lactic acid bacteria.
Abnormally high volatile acidity levels, however, are due to the breakdown of residual sugars, tartaric acid, and glycerol by anaerobic lactic acid bacteria. Aerobic acetic acid bacteria also produce acetic acid by oxidizing ethanol.
Finally, acescence in wine is linked to the presence of ethyl acetate, i.e. the ethyl ester of acetic acid, formed by the metabolism of aerobic acetic acid bacteria ( Section 2.5.1).
The fixed acidity of a wine is obtained by subtracting volatile acidity from total acidity. Total acidity represents all of the free acid functions, and volatile acidity includes the volatile acid functions, both free and in salt form. Strictly speaking, therefore, fixed acidity represents the free fixed acid functions plus the volatile acid functions in salt form (mainly acetic acid in salt form).
When fixed acidity is analyzed, there is a legal obligation to correct for sulfur dioxide and carbon dioxide. In practice, however, these two compounds have a similar effect on total acidity and volatile acidity, so the difference between total acidity and volatile acidity is approximately the same, with or without correction (Ribéreau‐Gayon et al ., 1982).
1.4 The Concept of pH and Its Applications
1.4.1 Definition
The concept of pH often appears to be an abstract, theoretical one, defined mathematically as the base ten logarithm of the concentration of hydronium ions in an electrically conductive solution, such as must or wine:
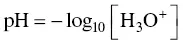
Furthermore, the expression of pH shows that it is an abstract measure with no units, i.e. with no apparent concrete physical significance.
The concepts of total or volatile acidity seem to be easier to understand as they are measured in milliliters of sodium hydroxide and expressed in grams of sulfuric or tartaric acid per liter. This is rather paradoxical as the total acidity in a wine is, in fact, a complex function with several variables, unlike pH, which refers to only one variable, the true concentration of hydronium ions in must and wine.
The idea that pH is an abstract concept is even less justified since this physicochemical parameter is based on the dissociation equilibrium of the various acids, HA, in wine, at fixed temperature and pressure, as shown below:
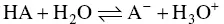
The emission of H 3O +ions defines the acidity of the HA molecule. Dissociation depends on the value of the equilibrium constant, K a, of the acid:
(1.1) 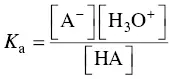
The usefulness of pH, also known as true acidity, is supported by the fact that its value rather accurately matches the impressions of acidity frequently described as “freshness” or even “greenness” and “thinness,” especially in white wines.
A wine's pH is measured using a pH meter equipped with a glass electrode after calibration with two buffer solutions. It is vital to check the temperature.
Owing to the presence of tartaric acid (a strong acid), the pH values of wines range from 2.8 to 4.0. It is surprising to find such low nonphysiological values in a biological fermentation medium such as wine. Indeed, life is only possible thanks to enzymes in living cells, and the optimum activity of the vast majority of enzymes occurs at much higher intracellular pH values, close to neutral, rather than those prevailing in extracellular media, i.e. must and wine. This provides some insight into the role of cell membranes and their ATPases in regulating proton input and output.
Читать дальше

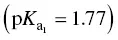 than carbonic acid
than carbonic acid 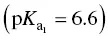 .
.