1 ...7 8 9 11 12 13 ...36 Wine color intensity is expressed as CI = OD420 + OD 520 + OD 620, or is sometimes expressed in a more simplified form: CI = OD 420 + OD 520.
Hue is expressed as
The total phenolic concentration is expressed by OD 280.
The analytical methods are described in Chapter 6of Volume 2.
PART I Chemistry of Wine
CHAPTER 1 Organic Acids in Wine
1 1.1Introduction
2 1.2The Main Organic Acids
3 1.3Different Types of Acidity
4 1.4The Concept of pH and Its Applications
5 1.5Tartrate Precipitation Mechanism and Predicting Its Effects
6 1.6Tests for Predicting Wine Stability
7 1.7Preventing Tartrate Precipitation
Organic acids make major contributions to the composition, stability, and organoleptic qualities of wines, especially white wines (Ribéreau‐Gayon et al ., 1982; Jackson, 1994). Their preservative properties also enhance wines' microbiological and physicochemical stability.
Thus, dry white wines that do not undergo malolactic fermentation are more stable in terms of bitartrate (KHT) and tartrate (CaT) precipitation. Young white wines with high acidity generally also have greater aging potential.
Red wines are stable at lower acidity, due to the presence of phenols that enhance acidity and help to maintain stability throughout aging.
1.2 The Main Organic Acids
1.2.1 Steric Configuration of Organic Acids
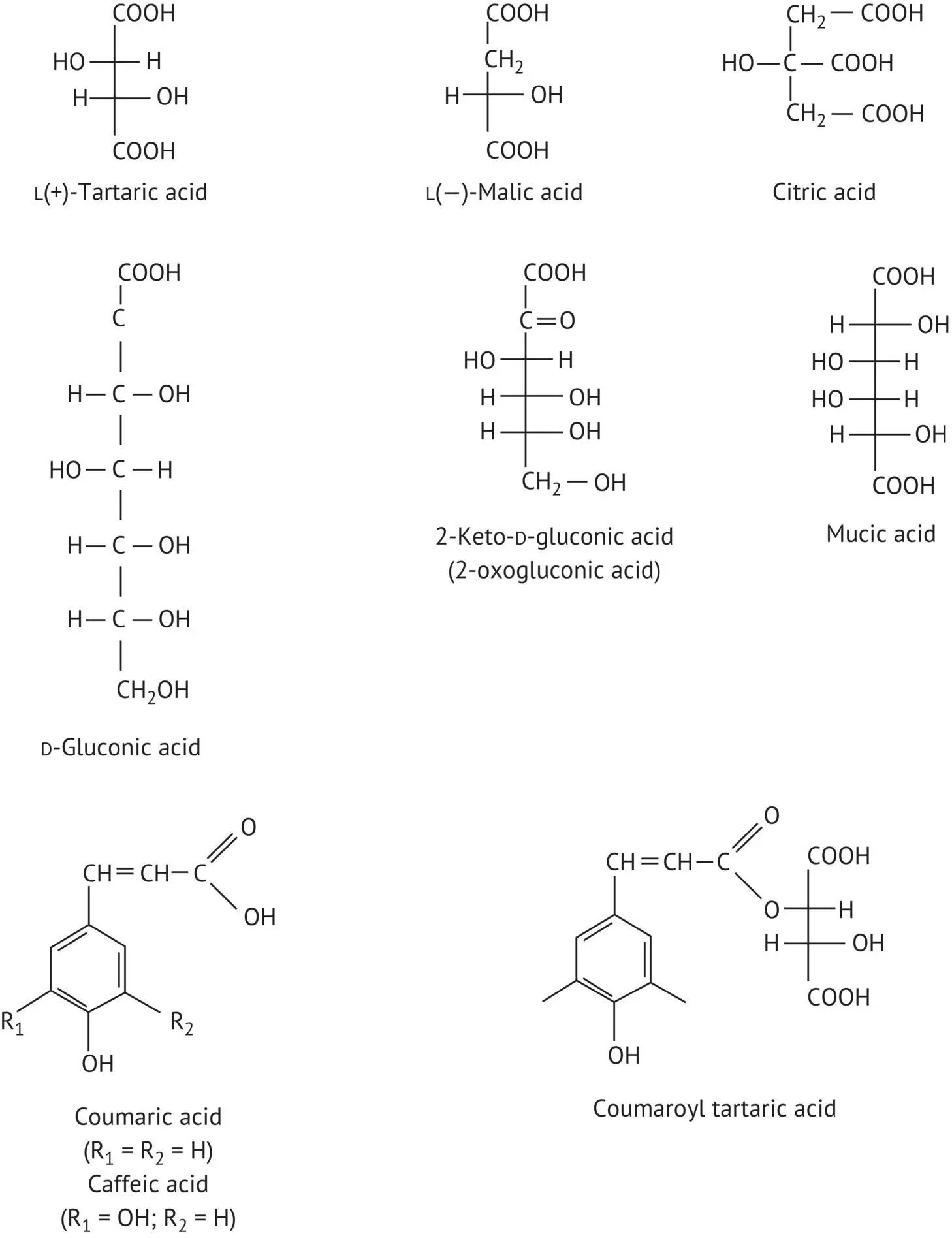
Most organic acids in must and wine have one or more chiral centers. The absolute configuration of the asymmetrical carbons is deduced from that of the sugars from which they are directly derived. This is especially true of tartaric and malic acids (Table 1.1). The absolute configuration of the asymmetrical carbons is established according to the Cahn–Ingold–Prelog priority rules (1953). Further reference to these rules will be made in the chapter on sugars ( Section 3.3.2), which are the reference molecules for stereoisomerism.
1.2.2 Organic Acids in Grapes
The main organic acids in grapes are described (Table 1.1) according to the conventional Fischer projection system. Grapes have a stereoisomer of tartaric acid in which the absolute configuration of the two asymmetrical carbons is L but whose optical activity in water, measured on a polarimeter, is D (or +). There is often confusion between these two concepts. The first is theoretical and defines the relative positions of the substituents for the asymmetrical carbon, while the second is purely experimental and expresses the direction in which polarized light deviates from a plane when it passes through the acid in a given solvent.
Tartaric acid is one of the most prevalent acids in must and wine. Indeed, at the end of the vegetative growth phase, concentrations in unripe grapes may be as high as 15 g/l. In musts from cool‐climate vineyards, concentrations are often over 6 g/l, whereas, in the warmer climates, they may be as low as 2–3 g/l, since combustion is more prevalent when the grape bunches are maintained at high temperatures.
Tartaric acid is not very widespread in nature, but rather is specific to grapes. For this reason, it is called Weinsäure in German, or “wine acid.” It is a relatively strong acid (see Table 1.3), giving wine a pH on the order of 3.0–3.5.
Tartrates originating from the wine industry are the main sources of tartaric acid, which is widely used in the food and beverage industry (soft drinks, chocolates, cakes, canned foods, etc.). This acid is also used for medical purposes (as a laxative) and in dyeing (for mordanting fabric), as well as for tanning leather. Tartrazine, a diazoic derivative of tartaric acid, is the yellow coloring matter in wool and silk but is also used as food coloring under the reference number E102.
L(−)‐Malic acid is found in all living organisms. It is especially plentiful in green apples, which explains its German name A˙pfelsäure , or “apple acid.” It is also present in white and red currants, rhubarb, and, of course, grapes. Indeed, the juice of green grapes, just before color change (veraison), may contain as much as 25 g/l. In the two weeks following the first signs of color change, the malic acid content drops by half, partly due to dilution as the grapes grow bigger and also as a result of combustion. At maturity, musts from cool climates still contain 4–6.5 g/l malic acid, whereas in warmer climates, concentrations are only 1–2 g/l.
Citric acid, a triacid, is very widespread in nature (e.g. lemons). Its very important biochemical and metabolic role (Krebs cycle, also known as the citric acid cycle) requires no further demonstration. Citric acid slows yeast growth but does not block it (Kalathenos et al ., 1995). It is used as an acidifying agent in the food and beverage industry (lemonade), while sodium (E331), potassium (E332), and calcium (E333) citrates have many uses in fields ranging from pharmaceuticals to photography. Concentrations in must and wine, prior to malolactic fermentation, are between 0.5 and 1 g/l.
In addition to these three acids, which account for the majority of the acidity in grapes, there are also phenol acids in the cinnamic series (e.g. coumaric acid), often esterified with an alcohol function of tartaric acid (e.g. coumaroyl tartaric acid).
Ascorbic acid ( Figure 1.1) should also be mentioned in connection with these oxidizable phenol acids. It is naturally present in lactone form, i.e. a cyclic ester. Ascorbic acid also constitutes a redox system in fruit juices, protecting the phenols from oxidation. In winemaking, it is used in conjunction with sulfur dioxide (Volume 1, Section 9.5).
TABLE 1.1 The Main Organic Acids in Grapes
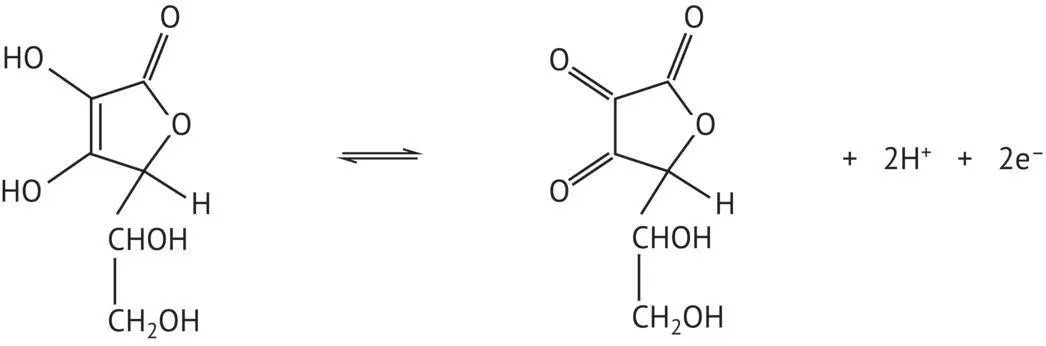
FIGURE 1.1 Oxidation–reduction equilibrium of ascorbic acid.
Must and wine from grapes affected by noble and/or gray rot have higher concentrations of acids produced by oxidation of the aldehyde function (e.g. aldose) or the primary alcohol function on C1 of a ketose (e.g. fructose). Thus, gluconic acid, the compound corresponding to glucose, may reach concentrations of several grams per liter in juice from grapes affected by mold. This concentration is used to identify wines made from grapes affected by noble rot as they contain less gluconic acid than those made from grapes affected by gray rot (Volume 1, Sections 10.6.4, 10.6.5 and 14.2.3). The compound corresponding to fructose is 2‐keto‐D‐gluconic acid (or 2‐oxogluconic acid) (Table 1.1).
The calcium and iron salts of these acids are used in medicine to treat decalcification and hypochromic anemia, respectively.
Calcium gluconate is known for its insolubility in wine and the turbidity it causes. Mucic acid, derived from galactose by oxidation, both of the aldehyde function of C1 and the primary alcohol function of C6, is just as undesirable. Also known as galactaric acid, it is therefore an aldaric acid. The presence of a plane of symmetry in its structure between C3 and C4 makes it a meso‐type stereoisomer. Mucic acid has no optical activity. Its presence has been observed in the crystalline deposits formed throughout the aging of sweet white wines made from grapes with noble rot.
Читать дальше