1 ...8 9 10 12 13 14 ...36
1.2.3 Organic Acids from Fermentation
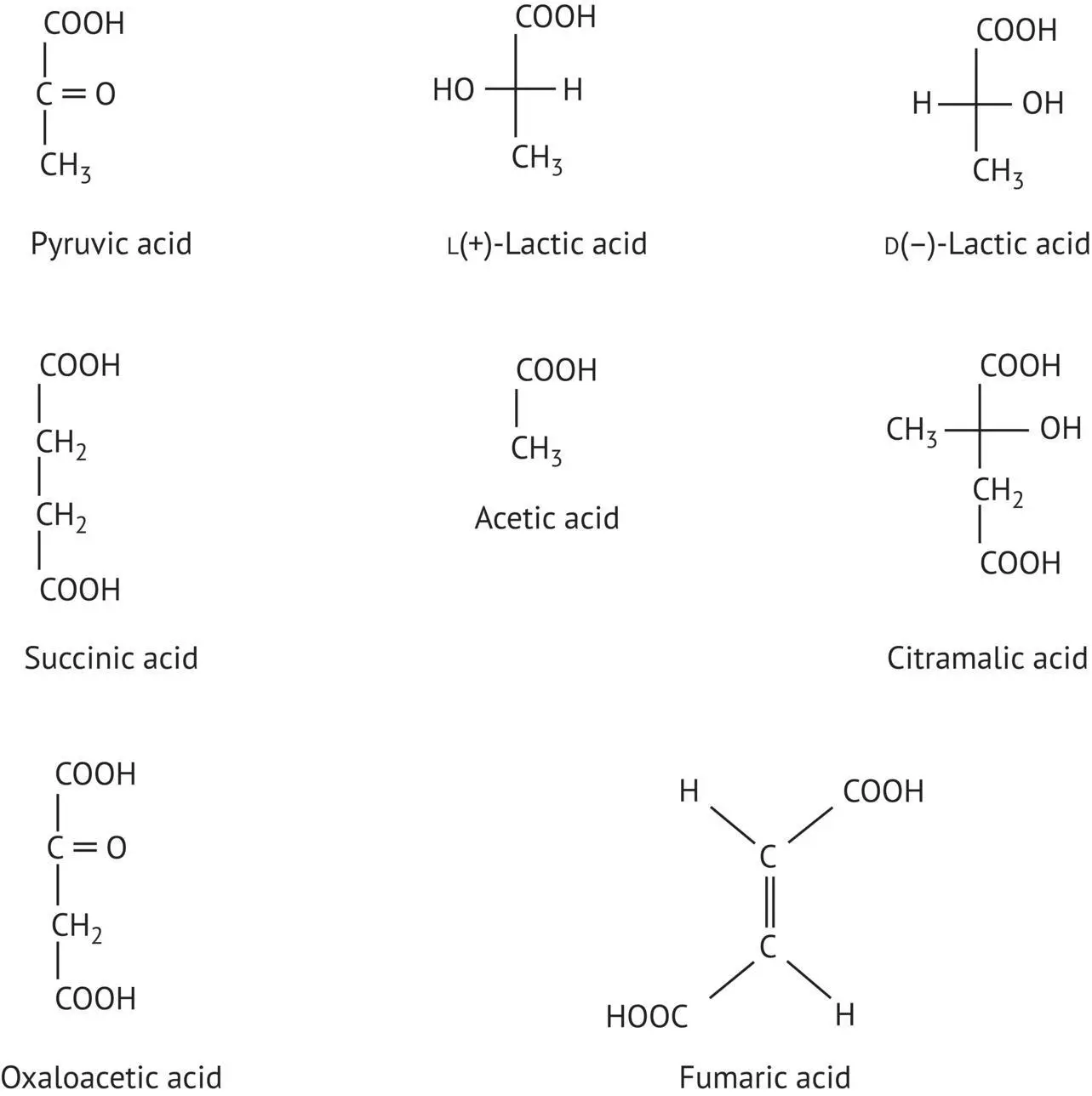
The main acids produced during fermentation are described in Table 1.2. The first to be described is pyruvic acid, due to its function as a “crossroad compound” in cell metabolism, although concentrations in wine are low, or even nonexistent. Following reduction by a hydride, or H −, ion (from aluminum or sodium borohydride) or by a coenzyme (NADH) from L- and D‐lactate dehydrogenases, pyruvic acid produces two enantiomers of lactic acid, L and D. The first dextrorotatory form is mainly of bacterial origin, and the second levorotatory form mainly originates from yeasts.
The activated, enol form of the same acid, phosphoenolpyruvate ( Figure 1.2) adds a nucleophile to carbon dioxide, producing oxaloacetic acid, a precursor of aspartic acid via transamination.
The enzymatic decarboxylation of pyruvic acid, assisted by thiamine (vitamin B1) pyrophosphate (TPP), produces acetaldehyde (ethanal), which is reduced to form ethanol during alcoholic fermentation. Its enzymatic, microbial or even chemical oxidation produces acetic acid.
Another acid that develops during fermentation due to the action of yeast is succinic, or 1‐4‐butanedioic, acid. Concentrations in wine average 1 g/l. This acid is produced by all living organisms and is involved in the lipid metabolism and the Krebs cycle, in conjunction with fumaric acid. It is a diacid with a high p K a( Table 1.3). Succinic acid has an intensely bitter, salty taste that causes salivation and accentuates a wine's flavor and vinous character (Peynaud and Blouin, 1996).
TABLE 1.2The Main Acids Produced During Fermentation
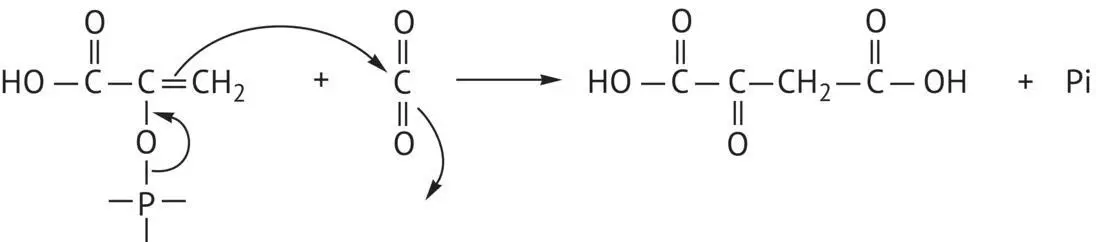
FIGURE 1.2 Biosynthesis of oxaloacetic acid from phosphophenolpyruvic acid.
Like succinic acid, citramalic acid, or α ‐methylmalonic acid, confused with citric acid in chromatography for many years, is of yeast origin.
In conclusion, it is apparent from this description that, independently of their origins, most of the main organic acids in must and wine consist of polyfunctional molecules, and many are hydroxy acids. These two radicals give these acids polar and hydrophilic characteristics. As a result, they are soluble in water and even in dilute alcohol solutions, such as wine. Their polyfunctional character is also responsible for the chemical reactivity that enables them to develop over time as wine ages. In this connection, results obtained by monitoring ethyl lactate levels in Champagne for two years after malolactic fermentation are highly convincing. Indeed, after two years of aging on the lees, concentrations reach 2 g/l and then decrease. The degree of acidity, indicated by the p K avalues of these acids, controls the extent to which they are present in partial salt form in wine ( Table 1.3).
A final property of the majority of organic acids in wine is that they have one or more asymmetrical carbons. This is a characteristic of biologically significant molecules.
TABLE 1.3State of Salification of the Main Inorganic and Organic Acids (Ribéreau‐Gayon et al., 1977)
| Category |
Name |
p K a |
Form in wine |
| Strong inorganic acids |
Hydrochloric |
Less than 1 |
Completely dissociated salts |
| Sulfuric 1 |
Approx. 1 |
| Sulfuric 2 |
1.6 |
|
| Sulfurous 1 |
1.77 |
Acidic bisulfite |
| Phosphoric 1 |
1.96 |
Acidic phosphate |
| Strongest organic acids |
Salicylic |
2.97 |
Acid functions partly neutralized and partly free (not highly dissociated) |
| Tartaric 1 |
3.01 |
| Citric 1 |
3.09 |
| Malic 1 |
3.46 |
| Formic |
3.69 |
| Lactic |
3.81 |
| Tartaric 2 |
4.05 |
| Weakest organic acids |
Benzoic |
4.16 |
Free acid functions (very little dissociated) |
| Succinic 1 |
4.18 |
| Citric 2 |
4.39 |
| Acetic |
4.73 |
| Butyric |
4.82 |
| Propionic |
4.85 |
| Malic 2 |
5.05 |
| Succinic 2 |
5.23 |
| Citric 3 |
5.74 |
| Weak inorganic acids |
Phosphoric 2 |
6.70 |
Free acid functions (almost entirely non‐dissociated) |
| Carbonic 1 |
6.52 |
| Sulfurous 2 |
7.00 |
| Hydrogen sulfide 1 |
7.24 |
| Carbonic 2 |
10.22 |
| Phosphoric 3 |
12.44 |
| Phenols |
Polyphenols (tannin and coloring) |
7–10 |
Free (non‐dissociated) |
1.3 Different Types of Acidity
The fact that enologists need to distinguish between total acidity, pH, and volatile acidity demonstrates the importance of the concept of acidity in wine. This is due to the different organoleptic effects of these three types of acidity. Indeed, in any professional tasting, the total acidity, pH, and volatile acidity of the wine samples are always specified, together with the alcohol and residual sugar contents.
The importance of total acidity is obvious in connection with flavor balance:

Looking at this balance, it is understandable that dry white wines have a higher total acidity than red wines, in which phenols combine with acids to balance the sweet taste of the alcohols. Volatile acidity indicates possible microbial spoilage.
Total acidity in must or wine, also known as “titratable acidity,” is determined by neutralization using a sodium hydroxide solution of known normality. The end point of the titration is still often determined by means of a colored reagent, such as bromothymol blue, which changes color at pH 7, or phenolphthalein, which changes color at pH 9. Using one colored reagent to define the end point of the titration rather than the other is a matter of choice. It is also perfectly conventional to use a pH meter and stop the total acidity assay of a wine at pH 7, and, indeed, this is mandatory in official analyses. At this pH, the conversion into salts of the second acid function of the diacids (malic and succinic) is not completed, while the neutralization of the phenol functions starts at pH 9.
The total acidity of must or wine takes into account all types of acids, i.e. inorganic acids such as phosphoric acid, organic acids including the main types described above, and amino acids whose contribution to titratable acidity is not very well known. The contribution of each type of acid to total acidity is determined by its strength, which defines its state of dissociation, as well as the degree to which it has combined to form salts. Among the organic acids, tartaric acid is mainly present in must and wine as a monopotassium acid salt, which still contributes toward total acidity. It should, however, be noted that must (an aqueous medium) and wine (a dilute alcohol medium), with the same acid composition and thus the same total acidity, do not have the same titration curve and, consequently, their acid–base buffer capacity is different.
Читать дальше