Yu Lan - Computational Methods in Organometallic Catalysis
Здесь есть возможность читать онлайн «Yu Lan - Computational Methods in Organometallic Catalysis» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Computational Methods in Organometallic Catalysis
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Computational Methods in Organometallic Catalysis: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Computational Methods in Organometallic Catalysis»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Computational Methods in Organometallic Catalysis — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Computational Methods in Organometallic Catalysis», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
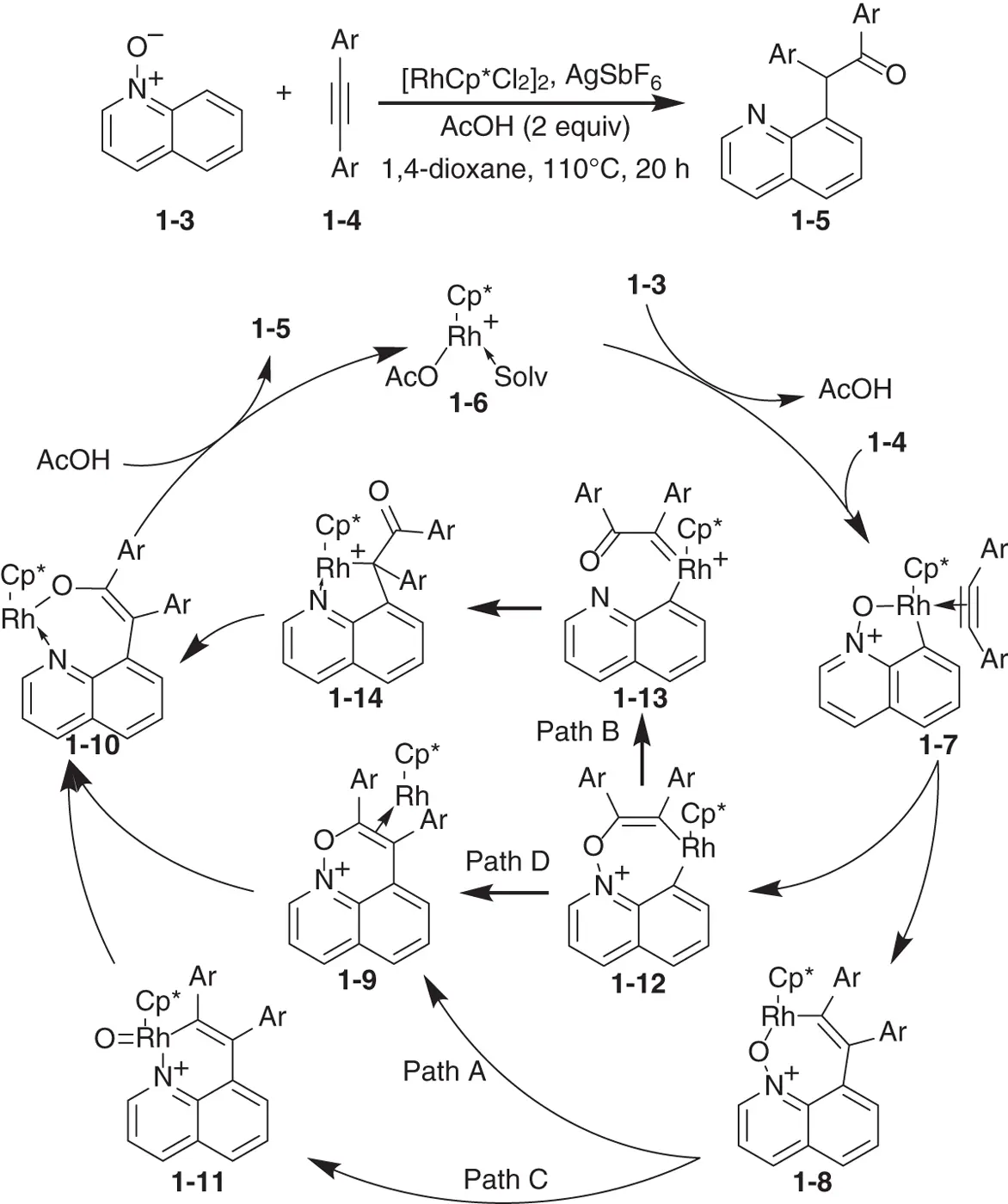
Scheme 1.6 Mechanism of rhodium‐catalyzed coupling reaction of quinoline N ‐oxide and acetylenes.
Source: From Li et al. [88].
In fact, quantum chemical calculations have become one of the conventional methods to study the mechanism of transition metal catalysis ( Scheme 1.7). The basis function of computational organometallic chemistry is to calculate the geometries and energies of stationary points, including local minimums and transition states, so as to construct potential energy surface for the catalytic cycle and explore the reaction mechanism. Information of molecular orbital, charge distribution, dipole moment, and so on can be achieved by computational population analysis, which is propitious to study factors of reactivity and selectivity. More useful information, including infrared, nuclear magnetic resonance, circular dichroism spectra, dispersion interactions, nucleophilicity/electrophilicity, and aromaticity, also can be achieved by theoretical calculations for organometallic chemistry.
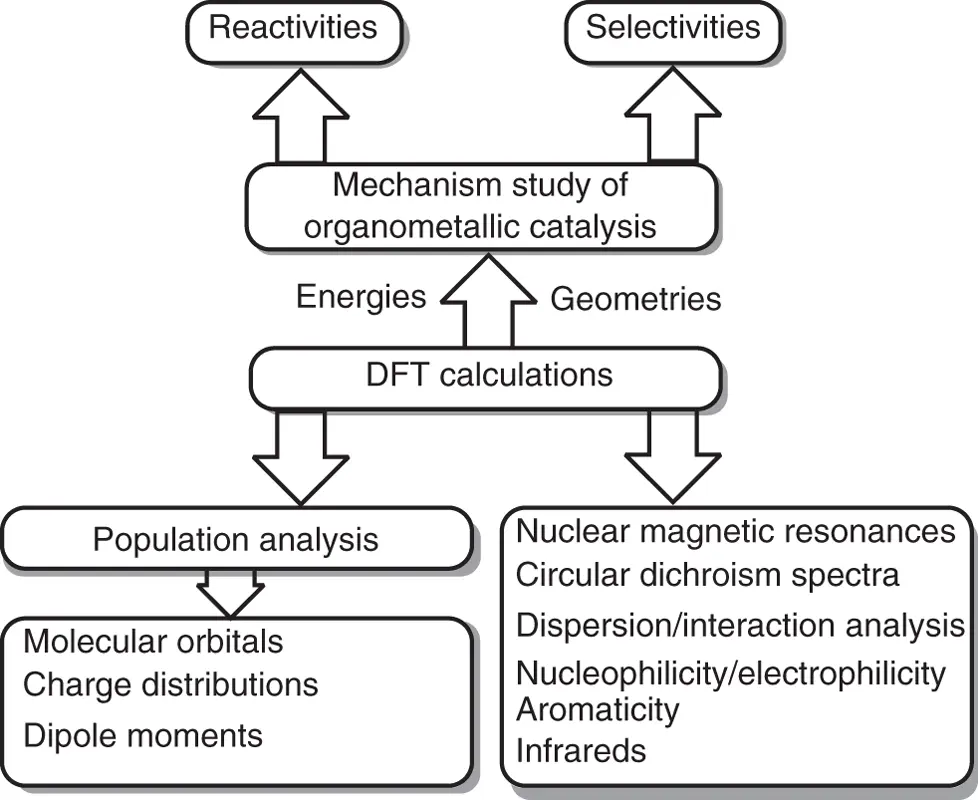
Scheme 1.7 Mechanism study of organometallic catalysis by density functional theory (DFT) calculations.
Over recent several decades, massive experimental and theoretical investigations were reported about organometallic catalysis. In those works, theoretical studies have proved to be indispensable routine technique for modern synthetic organic chemistry. Consequently, we herein provide a book to summarize and generalize the theoretical advances regarding organometallic catalysis. In Chapter 2, we summarize the popular computational methods, which are of benefit for the mechanism study of organometallic catalysis. We discuss the theoretical studies of elementary reactions in Chapter 3. Detailed processes for all the familiar elementary reactions in organometallic catalysis discovered by theoretical calculations are summarized in this chapter. Based on those two chapters, the second part of this book is organized by the type of transition metals, which are used as catalyst in organic reactions.
References
1 1 Olson, G.B. (2000). Designing a new material world. Science 288 (5468): 993–998.
2 2 Collins, T. (2001). Toward sustainable chemistry. Science 291 (5501): 48–49.
3 3 Feng, S. and Chien, S. (2003). Chemotherapeutic engineering: application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chemical Engineering Science 58: 4087–4114.
4 4 Thuronyi, B.W. and Chang, M.C.Y. (2015). Synthetic biology approaches to fluorinated polyketides. Accounts of Chemical Research 48: 584–592.
5 5 Xu, L., Zhang, S., Li, P. et al. (2015). Boron‐selective reactions as powerful tools for modular synthesis of diverse complex molecules. Chemical Society Reviews 44: 8848–8858.
6 6 Barboiu, M. and Gilles, A. (2013). From natural to bioassisted and biomimetic artificial water channel systems. Accounts of Chemical Research 46: 2814–2823.
7 7 Patel, M., Saunthwal, R.K., Verma, A.K. et al. (2017). Base‐mediated hydroamination of alkynes. Accounts of Chemical Research 50: 240–254.
8 8 Golder, M.R. and Jasti, R. (2015). Syntheses of the smallest carbon nanohoops and the emergence of unique physical phenomena. Accounts of Chemical Research 48: 557–566.
9 9 Kolb, H.C., Finn, M.G., Sharpless, K.B. et al. (2001). Click chemistry: diverse chemical function from a few good reactions. Angewandte Chemie International Edition 40: 2004–2021.
10 10 Mosesa, J.E. and Moorhousea, A.D. (2007). The growing applications of click chemistry. Chemical Society Reviews 36: 1249–1262.
11 11 Devaraj, N.D. and Weissleder, R. (2011). Biomedical applications of tetrazine cycloadditions. Accounts of Chemical Research 44: 816–827.
12 12 Hu, R., Leung, N.L.C., Zhong, B. et al. (2014). AIE macromolecules: syntheses, structures and functionalities. Chemical Society Reviews 43: 4494–4562.
13 13 Luo, Z., Yuan, X., Yu, Y. et al. (2012). From aggregation‐induced emission of Au(I)–thiolate complexes to ultrabright Au(0)@Au(I)–thiolate core–shell nanoclusters. Journal of the American Chemistry Society 134: 16662–16670.
14 14 Dreyer, D.R., Park, S., Bielawski, C.W. et al. (2010). The chemistry of graphene oxide. Chemical Society Reviews 1: 228–240.
15 15 Zou, X. and Zhang, Y. (2015). Noble metal‐free hydrogen evolution catalysts for water splitting. Chemical Society Reviews 44: 5148–5158.
16 16 Lee, Y.H., Zhang, X.Q., Zhang, W. et al. (2012). Synthesis of large‐area MoS2 atomic layers with chemical vapor deposition. Advanced Materials 24: 2320–2325.
17 17 König, H.M. and Kilbinger, A.F. (2007). Learning from nature: β‐sheet‐mimicking copolymers get organized. Angewandte Chemie International Edition 46: 8334–8340.
18 18 Biernacki, J.J., Bullard, J.W., Sant, G. et al. (2017). Cements in the 21st century: challenges, perspectives, and opportunities. Journal of the American Chemistry Society 100 (7): 2746–2773.
19 19 Long, N.J. and Williams, C.K. (2003). Metal alkynyl sigma complexes: synthesis and materials. Angewandte Chemie International Edition 42: 2586–2617.
20 20 Palacci, J., Sacanna, S., and Steinberg, A.P. (2013). Living crystals of light‐activated colloidal surfers. Science 339: 6122.
21 21 Lutolf, M.P. and Hubbell, J.A. (2005). Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnology 23: 47–55.
22 22 James, S.L. (2003). Metal–organic frameworks. Chemical Society Reviews 32: 276–288.
23 23 Hollister, S.J. (2005). Porous scaffold design for tissue engineering. Nature Materials 4: 518–524.
24 24 Hoyle, C.E. and Bowman, C.N. (2010). Thiol–ene click chemistry. Angewandte Chemie International Edition 49: 1540–1573.
25 25 Tan, C., Cao, X., and Wu, X. (2017). Recent advances in ultrathin two‐dimensional nanomaterials. Chemical Reviews 117: 6225–6331.
26 26 Larcher, D. and Tarascon, J.M. (2015). Towards greener and more sustainable batteries for electrical energy storage. Nature Chemistry 7: 19–29.
27 27 Ghidiu, M., Lukatskaya, M.R., Zhao, M.Q. et al. (2014). Conductive two‐dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 516: 78.
28 28 Cheng, F., Shen, J., Peng, B. et al. (2011). Rapid room‐temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts. Nature Chemistry 3: 79–84.
29 29 Duncan, R. (2003). The dawning era of polymer therapeutics. Nature Reviews Drug Discovery 2: 347–360.
30 30 Cho, S.H., Kim, J.Y., Kwak, J. et al. (2011). Recent advances in the transition metal‐catalyzed twofold oxidative C—H bond activation strategy for C—C and C—N bond formation. Chemical Society Reviews 40: 5038–5083.
31 31 Seregina, I.V. and Gevorgyan, V. (2007). Direct transition metal‐catalyzed functionalization of heteroaromatic compounds. Chemical Society Reviews 36: 1173–1193.
32 32 Fischbach, M.A. and Walsh, C.T. (2009). Antibiotics for emerging pathogens. Science 325: 1089–1093.
33 33 Shimizu, M. and Hiyama, T. (2005). Modern synthetic methods for fluorine‐substituted target molecules. Angewandte Chemie International Edition 44: 214–231.
34 34 Sun, J.Y., Zhao, X., Illeperuma, W.R.K. et al. (2012). Highly stretchable and tough hydrogels. Nature 489: 133–136.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Computational Methods in Organometallic Catalysis»
Представляем Вашему вниманию похожие книги на «Computational Methods in Organometallic Catalysis» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Computational Methods in Organometallic Catalysis» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.