Yu Lan - Computational Methods in Organometallic Catalysis
Здесь есть возможность читать онлайн «Yu Lan - Computational Methods in Organometallic Catalysis» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Computational Methods in Organometallic Catalysis
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Computational Methods in Organometallic Catalysis: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Computational Methods in Organometallic Catalysis»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Computational Methods in Organometallic Catalysis — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Computational Methods in Organometallic Catalysis», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Over recent decades, massive experimental and theoretical investigations on organometallic catalysis have been reported. In those works, theoretical studies have been proved to be an indispensable technique for modern organic chemistry. Consequently, this book is written to summarize and generalize the theoretical advances in the mechanistic study of organometallic catalysis. This book comprises two parts, which are the general overview of organometallic catalysis and the computational studies of reaction mechanisms classified by transition metals. I hope this book could inspire the mechanistic studies of complex reactions for theoretical chemists, and enable a better understanding of reaction mechanisms for experimental chemists.
29 July 2020
Yu LanZhengzhou, P. R. China
Part I Theoretical View of Organometallic Catalysis
It is time to write a book on computational organometallic chemistry.
The first part of this book can be considered as the introduction to computational organometallic chemistry. It is a long history since organometallic catalysis has been applied in organic synthesis; however, the mechanism of those reactions is too complicated to understand. Indeed, computational chemistry provided a powerful tool to reveal the mechanism of organometallic reactions. During recent two decades, the combination of computational chemistry and organometallic chemistry has made a series of progress in mechanistic studies, which has led to a new discipline, computational organometallic chemistry.
The first part would be composed of three chapters. In Chapter 1, a brief history of organometallics is given to reveal the significance of this chemistry. Computational chemistry, especially computational methods, is discussed in Chapter 2, which would be used in mechanism study of organometallic catalysis. Detailed processes for the familiar elementary reactions in organometallic catalysis discovered by theoretical calculations are summarized in Chapter 3.
1 Introduction of Computational Organometallic Chemistry
This chapter provides a brief introduction of computational organometallic chemistry, which usually focuses on the reaction mechanism of homogeneous organometallic catalysis.
1.1 Overview of Organometallic Chemistry
In this section, the historical footprint of organometallic chemistry is concisely given, which would help the readers better understand the role of computation in the mechanistic study of organometallic chemistry.
1.1.1 General View of Organometallic Chemistry
Creating new material is always entrusted with the important responsibility for the development of human civilization [1–3]. In particular, synthetic chemistry becomes a powerful tool for chemists, as it exhibits great value for the selective construction of new compounds [4–8]. Various useful molecules could be prepared by the strategies of synthetic chemistry, which provides material foundation, technological support, and drive force for science [9–20]. Synthetic chemistry is also the motivating force for the progress of material science, pharmaceutical science, energy engineering, agriculture, and electronics industry [21–41]. In this area, organic synthesis reveals broad interests from a series of research fields, which could target supply to multifarious functional molecules.
The synthetic organic chemistry usually focuses on “carbon” to widen related research, which could afford various strategies for the building of molecular framework, functional group transformations, and controlling stereochemistry in more sophisticated molecules [9, 22, 42–50]. Therefore, selective formation of new covalent bond between carbon atom and some other atom involving nitrogen, oxygen, sulfur, halogen, boron, and phosphorus becomes one of the most important aims for synthetic organic chemistry. In particular, nucleophiles and electrophiles are important for the construction of new covalent bonds.
A nucleophile, which is a molecule with formal lone‐pair electrophiles, can donate two electrons to its reaction partner for the formation of new covalent bond. Alternatively, an electrophile, which is a molecule with formal unoccupied orbitals, can accept two electrons from its partner for the formation of new covalent bond. Thereinto, coupling reactions could be categorized as redox‐neutral cross‐coupling with an electrophile and a nucleophile, oxidative coupling with two nucleophiles, and reductive coupling with two electrophiles ( Scheme 1.1).
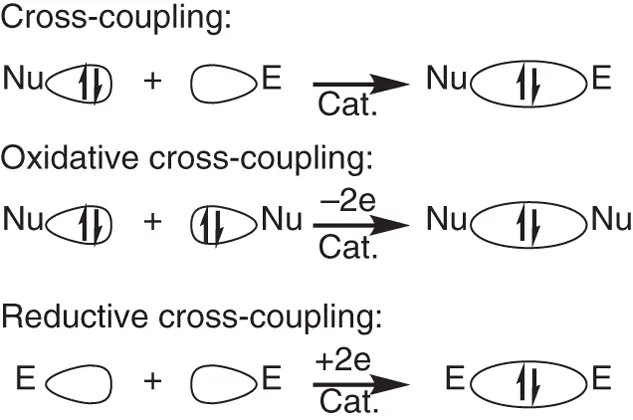
Scheme 1.1 Cross‐coupling reactions with nucleophiles and electrophiles.
In organic chemistry, the nucleophile is an electron‐rich molecule that contains a lone pair of electrons or a polarized bond, the heterolysis of which also could yield a lone pair of electrons ( Scheme 1.2). According to this concept, organometallic compounds, alcohols, halides, amines, and phosphines with a lone pair of electrons are nucleophiles. Some nonpolar π bonds, including olefins and acetylenes, which could donate the π‐bonding electrons, are often considered to be nucleophiles. Moreover, the C—H bonds of hydrocarbons can be considered to be nucleophiles because the electronegativity of carbon is higher than that of hydrogen, which could deliver a proton to form a formal carbon anion. Correspondingly, the electrophile is an electron‐deficient molecule that contains unoccupied orbitals or low‐energy antibonding molecular orbital, which could accept the electrons from nucleophiles. In this chemistry, cationic carbons, which usually come from the heterolysis of carbon—halogen bonds, are electrophile. Polar π bonds, including carbonyl compounds and imines, also could be considered to be electrophile, which involve a low‐energy π antibond. Interestingly, Fisher‐type singlet carbene has an electron pair filling one sp 2hybrid orbital and an unoccupied p orbital, which could be considered to be either nucleophile or electrophile in coupling reactions.
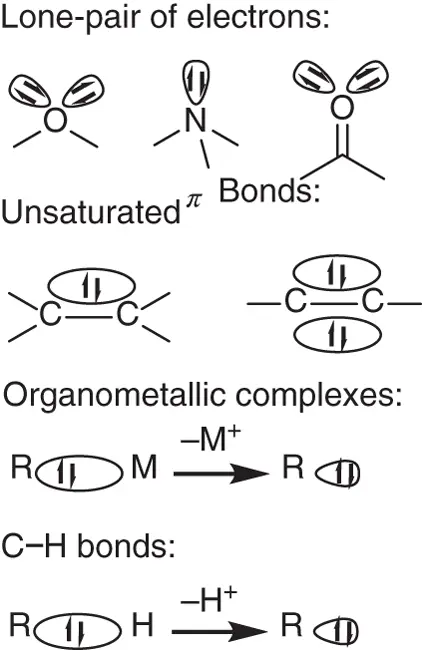
Scheme 1.2 Some selected examples of nucleophiles.
Superficially, at least, the reaction between nucleophile and electrophile could construct a covalent bond undoubtedly. However, the familiar nucleophiles and electrophiles, used in cross‐coupling reactions, are usually inactive, which could not react with each other rapidly. Moreover, when more active nucleophiles and electrophiles are used in coupling reactions, it would become out of control, which would not selectively afford target products. In effect, introducing transition metal catalysis can perfectly solve this problem. The appropriate transition metal can be employed to selectively activate the nucleophiles and electrophiles and stabilize some others, which led to a specially appointed cross‐coupling reaction.
High‐valence transition metal can obtain electrons from nucleophile, which led to the transformation of nucleophile into electrophile. The newly generated electrophile can couple with other nucleophiles to form covalent bond, which is named oxidative coupling reaction [51–53]. Meanwhile, the reduced transition metal can be oxidized by exogenous oxidant for regeneration. Correspondingly, low‐valence transition metal can donate electrons to electrophile leading to the transformation of electrophile into nucleophile, which can react with another electrophile to form covalent bond. Accordingly, it is named reductive coupling reaction. The oxidized transition metal also can be reduced by exogenous reductant.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Computational Methods in Organometallic Catalysis»
Представляем Вашему вниманию похожие книги на «Computational Methods in Organometallic Catalysis» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Computational Methods in Organometallic Catalysis» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.