1 ...6 7 8 10 11 12 ...24 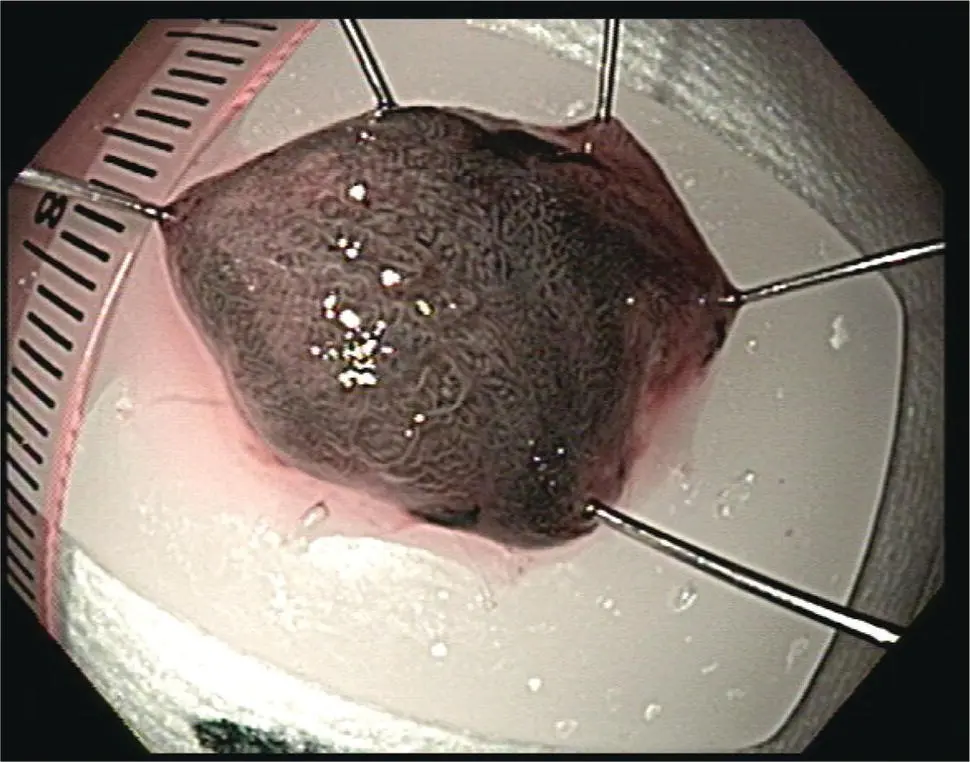
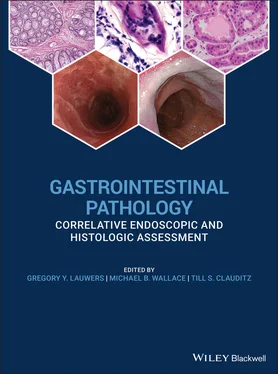
Figure 1.10 Pinned and oriented resection tissue from Barrett's esophagus‐associated neoplasia. Note the mucosal side facing up and the lateral margins pinned to prevent curling of the edges.
Samples obtained for culture should be placed in a sterile specimen container. Sterile saline maybe needed to prevent drying of the specimen. Attention should be paid to minimize contamination although it is recognized that the endoscope and the organs throughout which it is passed are not sterile and is not possible to obtain a purely sterile access into the gastrointestinal lumen. Contamination with oropharyngeal organisms or colonic organisms is not uncommon.
Specific Organ Sampling Methods
Esophagus
Diagnostic Sampling
Indications for diagnostic sampling of the esophagus include Barrett's esophagus and other suspected neoplastic disorders, inflammatory disorder such as eosinophilic esophagitis, gastroesophageal reflux disease, and infectious esophagitis.
The most common indication in Western countries for tissue sampling from the esophagus is likely Barrett's esophagus. There are established guidelines for sampling of the esophagus. These have traditionally been based on the notion that early neoplasia is not visible endoscopically and thus random sampling of the mucosa should be performed to ensure adequate detection of early neoplasia. The most widely used standard is the so‐called Seattle protocol. The American Society for Gastrointestinal Endoscopy (ASGE) guideline for tissue sampling calls for surveillance of nondysplastic Barrett's esophagus in four quadrants every 2 cm with a large‐capacity forceps for the entire Barrett's mucosa. Patients with established low‐grade dysplasia sampling should be more intensive with four‐quadrant biopsies every 1–2 cm. For patients who choose to undergo surveillance for high‐grade dysplasia, sampling every 1 cm should be performed; however, more recent evidence suggests that patients with established low‐grade and high‐grade dysplasia should likely undergo therapy to eradicate the Barrett's esophagus. Advances in endoscopic imaging, such as high‐definition narrow‐band imaging, confocal laser endomicroscopy, and chromoendoscopy, have significantly increased the yield of biopsy and allow much more targeted sampling although these have not yet replaced the need for random biopsy. These advances are likely to reduce the need for random biopsies and focus more on targeted sampling.
For eosinophilic esophagitis the ASGE recommends two to four biopsies from the proximal esophagus and two to four biopsies from the distal esophagus. Biopsy should also be obtained from the gastric antrum and duodenum when diffuse eosinophilic gastroenteritis is suspected.
For suspected infectious esophagitis, multiple biopsies from the margin and base of a visualized ulcer should be obtained and the sample should be sent for standard histology as well as immunohistochemical and possibly viral cultures and PCR. For candidal esophagitis, multiple biopsies of the affected area as well as cytology brushings may be complementary to biopsy.
Increasingly, endoscopic resection methods are being applied to early neoplasia the esophagus, particularly Barrett's esophagus and early squamous cell carcinoma. These are largely confined to tumors suspected to be T1 or nodular high‐grade dysplasia. Endoscopic mucosal resection (EMR) methods generally involve an EMR device such as a modified band ligator followed by snare resection of the pseudopolyp ( Figure 1.11– 1.13).
Other methods include endoscopic submucosal dissection (ESD) in which the lateral margins of the suspected area are incised with electrocautery knife followed by dissection along the submucosal plane to obtain an en bloc specimen. EMR devices can typically obtain samples 1–2 cm in diameter into the depth of the mid‐submucosa. Endoscopic submucosal dissection can obtain samples of any lateral diameter and generally to the base of the submucosa.
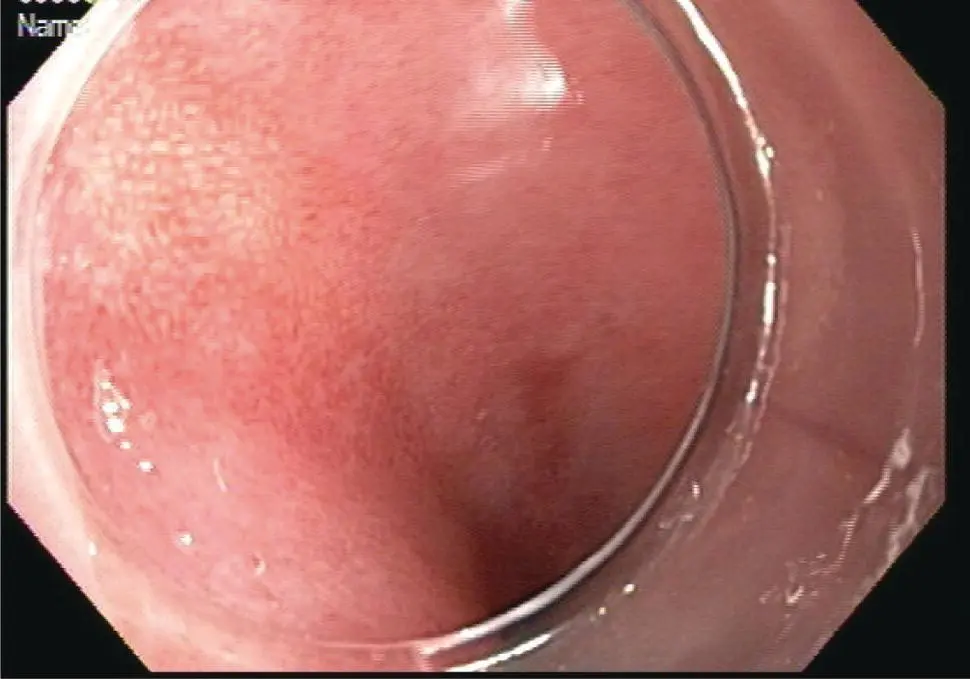
Figure 1.11 Barrett's esophagus with flat neoplasia (9 o'clock) for targeted endoscopic resection.
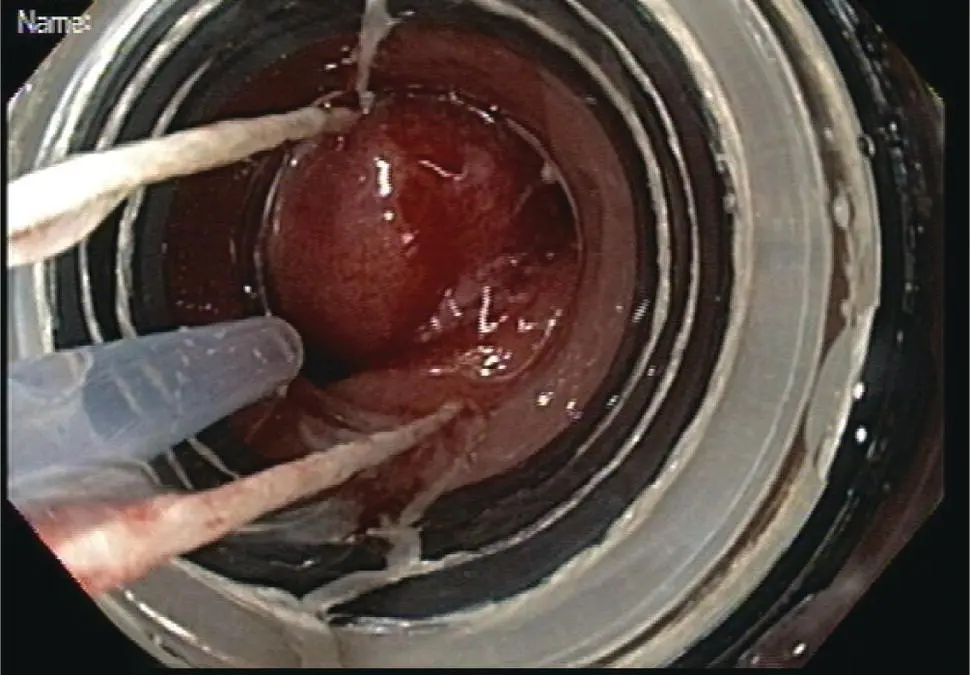
Figure 1.12Multiband mucosectomy device. The black rubber bands are mounted on the outside of a plastic cap. The tissue is suctioned into the cap and a band deployed to create a pseudopolyp, which is then removed by snare.
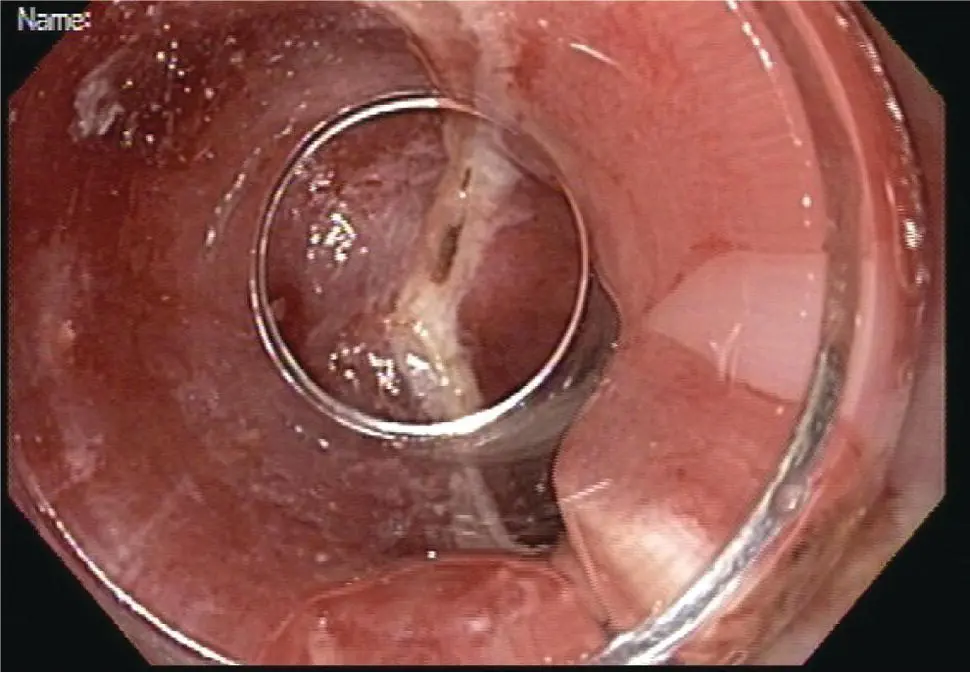
Figure 1.13 Area of resection at 6–12 o'clock includes the entire region of suspected neoplasia at 9 o'clock. The remaining tissue at the 9 o'clock area represents intact deep submucosa and muscularis propria.
One major advantage of these techniques is the ability to perform wide‐field or en bloc resection and orient the sample. Samples should be retrieved without causing trauma to the tissue, preferably by removal through the endoscopic cap or through a snare‐net as opposed to suctioning via the accessory channel, which can traumatize or fragment the tissue. Once retrieved the tissue should be handled as with other endoscopic resection specimens by orienting the specimen and pinning it on a fixed material such as a paraffin block. En bloc specimens can be oriented in terms of the oral and anal side of the lesion and assessed for lateral and deep margins. For resections that are performed piecemeal such as with multiband mucosectomy, the lateral margins cannot be accurately assessed and so complete resection relies on the endoscopic inspection intraluminally. The specimens should still be oriented and assessed for the deep margin.
Stomach
Diagnostic Sampling
Major indications for diagnostic sampling of the stomach include assessment for Helicobacter pylori infection, diagnosis of gastritis, metaplastic atrophic change, gastric polyps, and suspected neoplasia, particularly in the setting of gastric ulceration or early gastric cancer.
Biopsy is one of several recommended methods for H. pylori sampling that also includes urease breath testing and stool testing for H. pylori antigen. When using endoscopic tissue sampling, there are two general methods including non‐histological testing of the tissue for the presence of urease using the traditional Campylobacter‐like organism test (CLO‐test). In this case, the tissue should be placed in the standard agar well and visually inspected for a pH change following the instructions for use in this product. Histological sampling can also be performed with one of two methods. One method is to take three biopsies including one from the angularis corpus antrum junction, one from the greater curvature of the corpus, and one from the greater curvature of the antrum. Alternatively, the updated Sydney protocol may be followed, which includes five biopsies including one from the antrum lesser curve, antrum greater curve, gastric corpus lesser curve, and greater curve, and one from the angularis of the stomach.
Читать дальше