Another variant of SPME which has also been applied for PAEs extraction is in-tube (IT)-SPME [60]. In this format, a very thin tube is coated in its inner walls and the extraction and desorption are carried by the introduction and extraction of the sample inside the tube several times [61]. As in conventional SPME, the combination of materials with high surface areas and polymers afford a high extraction capability, while its porous structure provides suitable dynamic transport during extraction. As examples, Wang et al. [23] used poly(dopamine) (PDA) to functionalize melamine formaldehyde aerogel on carbon fibers and were packed inside IT-SPME tubes for the extraction of seven PAEs from drinkable and surface water, while the performance of this process embedding activated carbon (without any chemical modification) in different polymers (e.g., poly(butyl methacrylate-co-ethylene dimethacrylate) (poly(BMA-EDMA)) and PS-DVB) was investigated by Lirio et al. [62]. In this last work, low extraction recovery values were obtained when a solution containing eight PAEs was collected using monolithic columns with native poly(BMA-EDMA) and PS-DVB. On the contrary, the presence of increasing amounts of activated carbon provided a higher extraction efficiency under the same conditions. Moreover, the activated carbon-PS-DVB monolithic column exhibited better extraction performance than the activated carbon-poly(BMA-EDMA) one. Therefore, the first was applied in the IT-SPME of mineral water samples obtaining recovery values in the range of 78.8%–104.6% at 50 μg/L.
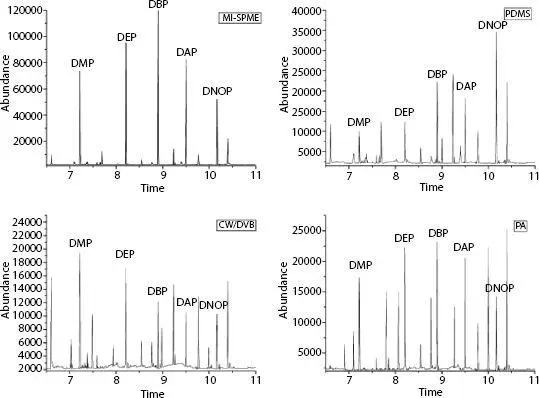
Figure 1.4Extraction yields with different fibers (MI-SPME, PDMS, CW/DVB, and PA) in water samples. Extraction conditions: 12 ml of spiked pure water including NaCl content of 10% w/v, stirring at 60°C in DI, adsorption time 30 min, desorption at 250°C for 10 min. Reprinted from [45] with permission from Elsevier. CW, carbowax; DAP, diallyl phthalate; DBP, dibutyl phthalate; DEP, diethyl phthalate; DMP, dimethyl phthalate; DNOP, di-n-octyl phthalate; DVB, divinylbenzene; MI, molecular imprinted polymer; PA, polyacrylate; PDMS, polydimethylsiloxane; SPME, solid-phase microextraction.
1.3 Stir Bar Sorptive Extraction
As a technique derived directly from SPME, SBSE is based on the same principles of distribution of the analytes between the sorbent and the sample. This technique, introduced by Baltussen et al. [63], is based on the use of a small device consisting of a magnetic bar which is introduced in a glass tube coated by PDMS in most cases, generally using around 50–300 times larger PDMS amounts as coating, so SBSE significantly increases the enrichment factors compared to SPME, providing at the same time a higher extraction efficiency as a consequence of its larger volume and surface area. Moreover, SBSE desorption can be directly developed in a thermal desorption unit in many GC systems, so it maintains one of the main advantages derived from the use of SPME [64]. Since then, this technique has evolved and a wide variety of laboratory made coatings have been developed in order to extend the field of application of SBSE, especially to the extraction of polar compounds, as well as to improve its selectivity [64]. Up to now, few works have used SBSE for the determination of PAEs in water samples (see Table 1.1). This is the case of the work of Prieto et al. [65], who analyzed six PAEs together with sixteen polycyclic aromatic hydrocarbons (PAHs), twelve polychlorinated biphenyls (PCBs) and three nonylphenols in sea and estuarine waters, and that of Si et al. [66], who extracted fifteen PAEs from sea water. In both works, commercial PDMS-coated stir bars of 0.5- and 1-mm thickness were used, respectively. In both cases, the addition of methanol (MeOH) to the water sample (20% for 0.5-mm thickness fibers and 10% for 1-mm thickness fibers, v/v) was crucial to avoid PAEs adsorption on the glass walls and to improve the extraction efficiency, particularly for long chain PAEs. In the last work, four commercially available stir bars containing different amounts of PDMS were evaluated in terms of extraction capacity. The results showed that the stir bar containing the highest amount (150 μL vs. 50, 75, and 150 μL over carbon) provided the largest peak areas, which was consistent with the principles of the process. However, the sensitivity for the higher-molecular weight PAEs (i.e., DNOP and DEHP) was notably lower, which is probably caused by the fact that this kind of nonpolar compounds establish strong interactions with the PDMS coating which could result in an incomplete desorption and the consequent higher LODs and LOQs. The combination of SBSE with GC-MS systems using single quadrupoles operated in the SIM mode allowed obtaining high extraction efficiency as well as high sensitivity (0.1–489 ng/L) in general.
1.4 Solid-Phase Extraction
One of the most important advantages of SPE compared to SPME is the availability of a wider range of relatively cheap commercial sorbents. However, the low rate of diffusion and mass transfer between the packed sorbents and the target analytes generally results in a slow extraction process. Moreover, in some cases, cartridges can be blocked when complex matrices are analyzed, leading to process failure. Besides, a previous cartridge conditioning is required which also lengthens the extraction process. In order to solve these problems, dSPE—as a miniaturized version of SPE in many cases—emerged as an alternative based on the direct addition of the sorbents (without preconditioning) to samples or extracts [67, 68], improving the contact area between sorbent and the sample/extract solution. Consequently, the analytes are extracted much faster by simple manual, vortex, or ultrasound agitation, while most interferences remain in the solution (depending on the selectivity of the sorbent). Then, the sorbent can be separated by its retention into a SPE column [25] or by centrifugation and the subsequent supernatant decantation [69]. In the first case, the analytes are later eluted as in conventional SPE, while in the second, the analytes have to be desorbed under agitation, centrifugation, and decantation. Therefore, this variant saves time, efforts, and solvents, with the consequent economic savings that this entails [70, 71]. The few dSPE methods developed for the extraction of PAEs from water samples (see Table 1.2) have been based on the application of graphene [72], GO-MIP [69], MIPs microbeads [73], a commercial metal-organic framework (MOF) [25], and a temperature-sensitive polymer [74]. In this last case, a lab-synthesized β-cyclodextrin-poly(N-isopropylacrylamide) (β-CD-PNIPAM) polymer was used to carry out the extraction of three short-chain PAEs from tap and mineral water. This kind of polymers are characterized by showing a reversible phase transition in aqueous samples at a certain temperature known as low critical solution temperature (LCST). In this case, the LCST is at 32°C, so the polymer behaves as a liquid below that value while it is a solid when the temperature exceeds it. Taking advantage of this fact, the authors introduced 20 mL of β-CD-PNIPAM into the sample and mixed. Then, the temperature was increased up to 50°C obtaining a floating solid phase, which was collected after Na 2SO 4addition in order to favor the salting-out effect. This extraction methodology, in combination with GC-MS, allowed obtaining good extraction efficiency and sensitivity, especially for DBP, which makes think about the potential of this procedure for the determination of long-chain PAEs. It is important to mention that, from an operational point of view, the use of this kind of polymers implies that the extraction step is developed in the same way as a LLE procedure and, as consequence, it could be not considered as a sorbent-based extraction methodology for some authors. However, the second part involve a desorption of the analytes from the solid polymer, since that reversible phase transition just takes place when it is into the water sample. In order to provide a better vision of this procedure, Figure 1.5 shows a scheme of a similar procedure used by Chen et al . in a previous work for the determination of different phenolic compounds—no PAEs were included [75].
Читать дальше