3.2 Carl Scheele, Joseph Priestley, and the discovery of oxygen
Carl Scheele (1742–1786) was a Swedish chemist who was almost certainly the first person to isolate oxygen, producing it chemically in about 1771. Unfortunately, Scheele did not publish his results until 1777, well after both Priestley and Lavoisier had published their work. Their findings were at least partially based on Scheele's results, which he had communicated to them. Scheele also died at an early age of 44, almost certainly poisoned by some of the chemicals that he worked with, which he liked to taste and sniff.
Joseph Priestley (1733–1804) made many important discoveries, especially in relation to the properties and handling of gases. Priestley was an eighteenth‐century English country minister who had a passion for science (Jaffe, 1976). His formal education was limited primarily to theology and languages, whereas his scientific knowledge was mostly self‐taught. He was deeply influenced by a meeting with Benjamin Franklin, whom he met on a trip to London. He lived next to a brewery in Leeds, which provided a constant supply of carbon dioxide, so initially, he studied that gas's properties. One of his first results was the invention of seltzer water, for which the British Royal Society awarded him a gold medal.
We remember Priestley especially for his discovery of oxygen, first in an indirect way, by observing the action of plants in 1771, and then in pure form in 1774 by heating mercuric oxide and collecting the gas given off. He described the 1771 experiments thus:
Finding that candles would burn very well in air in which plants had grown a long time … I thought it was possible that plants might also restore the air which has been injured by the burning of candles. Accordingly, on the 17th of August, 1771, I put a sprig of mint into a quantity of air in which a wax candle had burned out and found that on the 27th of the same month another candle burnt perfectly well in it.
Priestley's interpretation of this and related experiments ( Fig. 3.1) was that the candle (or mouse – he had a plentiful supply of mice and often used them as test subjects) produced large amounts of phlogiston, which was the basis for interpreting all chemical processes at that time. Phlogiston, which is derived from the Greek word for “to set on fire,” was thought to be a flammable substance possessed by all substances that can burn. Upon combustion or respiration, the phlogiston was released into the air and contaminated it. Plants had the unique ability to recapture the phlogiston that had been released by burning. In his later experiments, Priestley was able to prepare and analyze in some detail significant quantities of pure oxygen, which he called “vital air.” Priestley was a brilliant experimentalist, whose abilities to generate and manipulate gases were far better than those of most of his contemporaries. However, his skills at interpreting the observations he made were not as strong. He interpreted all his observations in terms of the phlogiston theory, even after most other scientists had abandoned it. He was usually content to simply describe his results with little interpretation. Priestley traveled to France in 1774 and met with Lavoisier, to whom he openly described his many experiments. Lavoisier used this information, as well as many of his own experiments, to overturn the phlogiston theory and establish the modern study of chemistry.
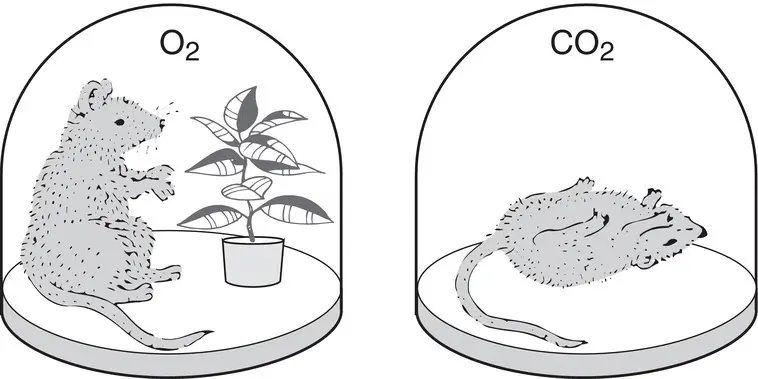
Figure 3.1 Joseph Priestley and the discovery of oxygen.
Priestley was both a religious and a political nonconformist and vocally supported both the American and the French revolutions. He was often in trouble for his unorthodox views, was attacked by the press and denounced in Parliament, and the scientific establishment increasingly shunned him. In 1791, an angry mob burned his house to the ground. In 1794, he fled to the USA and settled in Pennsylvania, where he was warmly welcomed by both religious and scientific leaders. He lived there until he died in 1804.
3.3 Ingenhousz and the role of light in photosynthesis
Jan Ingenhousz (1730–1799) was a Dutch physician and man of the world; he was at one‐time court physician to the Austrian empress Maria Theresa and had also visited England on several occasions (Magiels, 2010). On one of these trips in 1773, he heard a lecture by John Pringle, the President of the Royal Society, in which he presented a medal to Priestley. In his lecture, Pringle lauded Priestley's accomplishments. Ingenhousz thus became interested in the subject, but took no action until several years later when, in 1779, he rented a house in the English countryside and in the short space of three months conducted more than 500 experiments on the properties of plants and their effects on the air. During this frenzied summer, Ingenhousz discovered the essential role of light in the process of photosynthesis. By October of the same year, he had already written up his results in the form of a book entitled Experiments upon Vegetables, Discovering Their Great Power of Purifying the Common Air in Sunshine and Injuring It in the Shade and at Night . In addition to discovering the effect of sunlight, Ingenhousz discovered plant respiration, although he greatly overemphasized the effects of it, as can be seen from the title of his book. Ingenhousz was a brilliant, but not very careful, scientist and often jumped to conclusions with little evidence. Nevertheless, his contributions to the understanding of photosynthesis were extremely important, including a book published in 1796, Food of Plants and Renovation of the Soil . In this book, he summarized and presented for the first time the process of photosynthesis in terms of the new chemistry proposed by Lavoisier.
3.4 Senebier and the role of carbon dioxide
Jean Senebier (1742–1809) was a Swiss minister, contemporary, and bitter rival of Ingenhousz. His most important contribution to the developing understanding of photosynthesis was his finding in 1783 of the essential role that carbon dioxide, or “fixed air,” has in the process. Senebier and Ingenhousz engaged in a long‐standing and acrimonious priority dispute. This was not helped by Senebier's poor writing style, such that he would take entire volumes to describe his results in tedious detail, with no summaries provided. For his part, Ingenhousz tried to claim in his 1796 book that he had actually discovered the role of carbon dioxide in his 1779 experiments, although he had clearly denied that it was important in writings in 1784.
3.5 De Saussure and the participation of water
The final contribution to the overall equation of photosynthesis was made by Nicolas de Saussure (1767–1845), a Swiss scientist. He confirmed the observations of Ingenhousz and Senebier and added careful measurements of the relative amounts of carbon dioxide taken up and oxygen given off by plants. He proved by careful weighing experiments, published in 1804, that the increase in dry weight of the plant is considerably greater than the weight of carbon in the carbon dioxide taken up. He correctly surmised that the balance of the weight came from water, although he mistakenly thought that the assimilation of water was a process separate from the incorporation of carbon dioxide.
3.6 The equation of photosynthesis
The period between 1771 and 1804 was exciting in the history of photosynthesis. In this short time, the basic chemical equation of photosynthesis was established, which in 1804 could be written as:
Читать дальше













