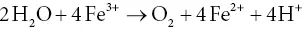3.7.2 The Hill reaction: separation of oxidation and reduction reactions
In the 1930s, Robert (Robin) Hill (1899–1991), working at Cambridge University, was able to separate and investigate individually the oxidation and reduction reactions of photosynthesis. This taking apart of a complex system into individual parts, and investigation of them in detail in the absence of the complexities of a living cell is a cornerstone of biochemistry, often called reductionism. Hill established that it was possible to restore high rates of oxygen evolution to chloroplast suspensions if the latter were supplied with any of a number of artificial electron acceptors (Hill, 1939). Initially, he used ferric salts, and the Fe 3+was reduced to Fe 2+through the action of light, at the same time producing O 2. The reduction in artificial acceptors with concomitant O 2production is today known as the Hill reaction. An example of the Hill reaction is given in Eq. (3.6):
(3.6) 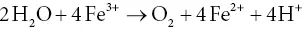
Hill measured the O 2in an ingenious way, which is worth relating if only to give an idea of the remarkable advances made by many of the pioneers of the field despite their primitive instrumentation. Hill obtained whole blood from a slaughterhouse, which has a dark blue color when deoxygenated and bright red color when oxygenated. He combined this with his chloroplast preparation and illuminated the mixture, monitoring the degree of oxygenation of the blood using a hand‐held spectroscope. At first, the results were disappointing, because the sample produced little oxygen. It is now clear that this was because the outer chloroplast envelope membranes were broken during the preparation, and the enzymes needed for CO 2assimilation were lost. In searching for the factors needed to restore the lost activity, Hill made a fundamental discovery: namely, that it was possible to replace the reduction of CO 2with the reduction of artificial electron acceptors, thereby restoring high rates of O 2production. The physiological compound that acts as the light‐driven electron acceptor facilitating CO 2production is NADP +, the oxidized form of nicotinamide adenine dinucleotide phosphate. The reduced form of this compound, NADPH, then serves as the reductant for CO 2assimilation.
Hill did not set out to discover the reaction that bears his name. Instead, he was trying to establish whether an isolated chloroplast was capable of the complete process of photosynthesis, which was an important issue at the time. In fact, it is quite difficult to isolate chloroplasts with the envelope membranes still intact, and this was not routinely achieved until the mid‐1960s. This is a good example of Louis Pasteur's famous saying that “fortune favors the prepared mind.”
3.8 The Emerson and Arnold experiments
In 1932, Robert Emerson (1903–1959) and his undergraduate research student at the California Institute of Technology, William Arnold (1904–2001), published two seminal papers. They were the first scientists to exploit the use of very short flashes of light to probe photosynthesis. At the time, this was extremely difficult technically; one did not just order a flash instrument from a scientific supply house. Emerson and Arnold built their own apparatus from automobile ignition points, capacitors, neon lights, and even a hot plate (which served as a ballast resistor) (Myers, 1994). Using this device, they were able to obtain flash durations as short as 10 μs. Emerson and Arnold utilized the green alga Chlorella pyrenoidosa as their experimental organism and measured oxygen evolution using manometers, U‐shaped tubes containing liquids in which the level was used to determine the volume of gas produced or consumed. Emerson was a master in the techniques of manometry, which he had learned from his former mentor, Otto Warburg, and could measure minute changes of O 2with great accuracy.
In the first series of experiments (Emerson and Arnold, 1932a), they varied the time between flashes and found that if there was a long time between flashes, then the yield of O 2per flash was independent of the time between flashes and did not depend on temperature between 1 and 25 °C ( Fig. 3.2). With shorter times between flashes, the yield fell off dramatically at the lower temperature, but did not change at the higher temperature. This result was elegantly interpreted as evidence that photosynthesis involved both a light stage and a dark stage. The light stage, which we now refer to as a photochemical reaction, can happen extremely rapidly and is not dependent on temperature. The dark stage, which we now know to be a series of enzymatic reactions, is slower and, like most chemical reactions, depends on temperature activation to proceed. These results, which were partially anticipated by other scientists using continuous and crude intermittent light methods, were readily interpretable on the basis of the understanding of photosynthesis available at the time. However, they did no more than set the stage for the remarkable result that Emerson and Arnold found in their second set of experiments, in which they examined the light‐intensity dependence of the photochemical reaction.
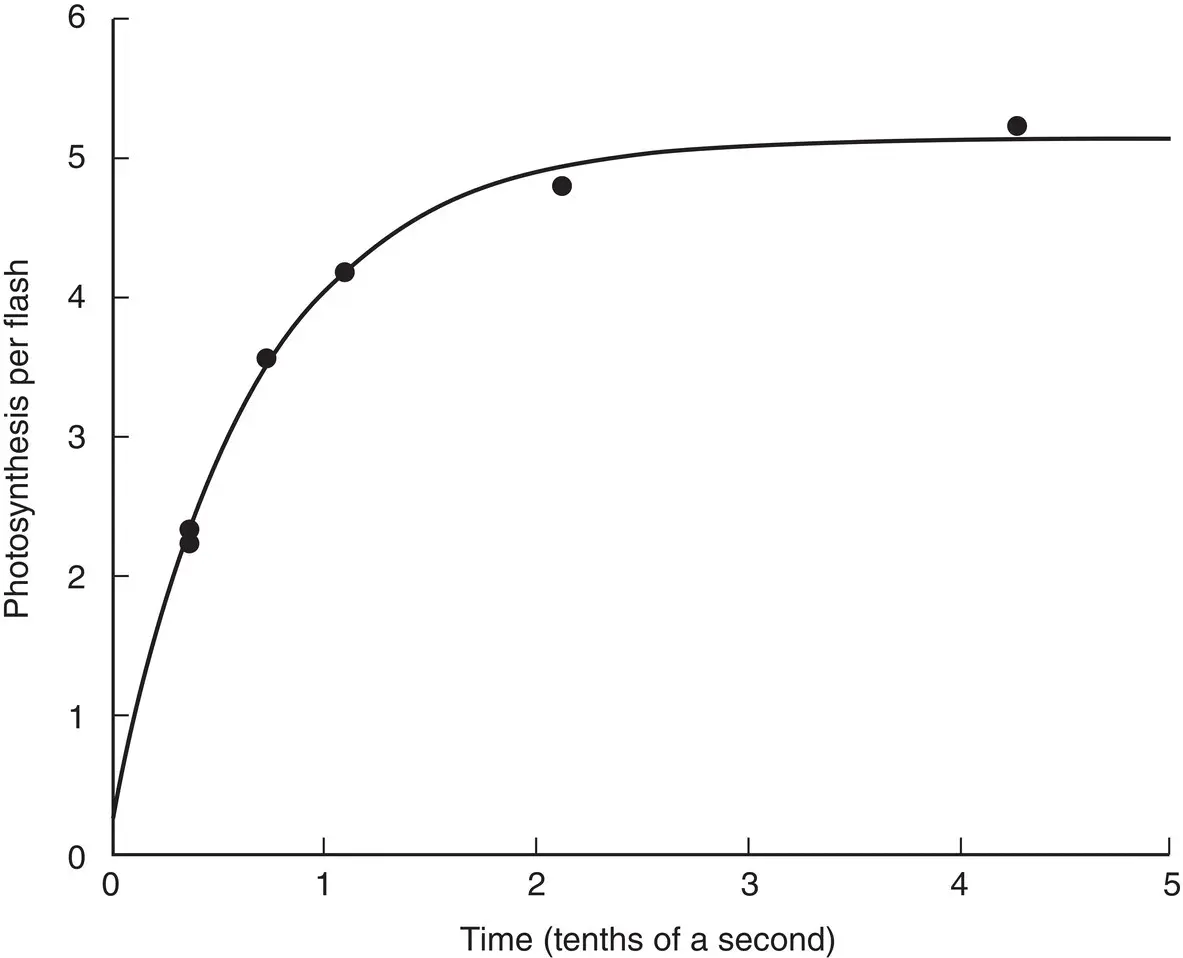
Figure 3.2 Emerson and Arnold's experiment establishing a light stage and a dark stage of photosynthesis. Time refers to the time between flashes.
Source: Emerson and Arnold (1932a)/Rockefeller University Press.
For the second series of experiments, Emerson and Arnold (1932b) varied the light intensity of the flashes, using a flash spacing that they knew from the first series of experiments was long enough for the dark enzymatic reactions to proceed to completion. This experimental protocol enabled them to isolate the photochemical reaction and study it without interference from the later steps. At very low intensities the yield of O 2per flash was low and depended linearly on the flash energy. However, at higher intensities, the curve saturated, so additional flash energy gave no additional O 2( Fig. 3.3).
Most experimenters would have been satisfied with just establishing that the curve saturated at a high light intensity. After all, if at very high light intensity every chlorophyll molecule absorbs a photon and produces photoproducts, then one expects that additional light will give no further products until the slow enzymatic reaction restores the chlorophyll to an active state. The beauty of the experiment lies in the fact that Emerson and Arnold took great pains to obtain a quantitative measure of how much O 2was produced per chlorophyll in the sample. This may sound like a simple matter, but at the time the quantitative absorption properties of chlorophyll were not well known, so Emerson and Arnold had to determine this in order to know how many chlorophyll molecules were in their sample. The measurement of the amount of O 2produced was easier, utilizing the resulting volume and the known properties of gases.
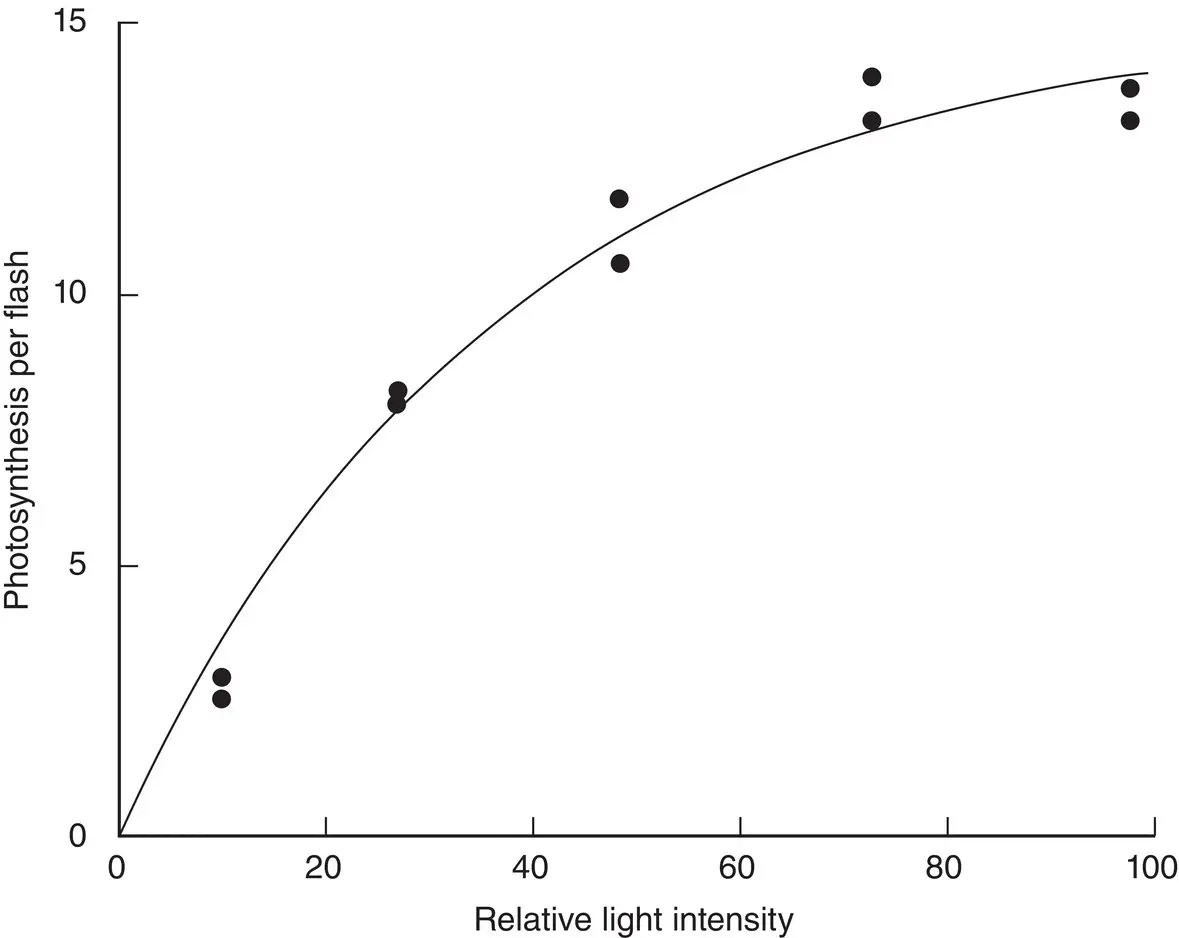
Figure 3.3 Emerson and Arnold's experiment establishing the light saturation curve for photosynthesis in flashing light.
Source: Emerson and Arnold (1932b) (p. 1940)/Rockefeller University Press.
The final result was a huge surprise. Only one O 2was produced for every 2500 chlorophyll molecules, far less than the one per chlorophyll that was expected! Their findings were difficult to reconcile with the common idea at the time that each chlorophyll molecule directly reduced CO 2. It was many years before the true significance of this experiment was appreciated. We now know that the vast majority of chlorophyll molecules act as antennas and function only to collect light, transferring the energy to a special chlorophyll molecule (which is part of a protein complex known as the reaction center) that actually does the photochemistry. This is like a satellite dish, which collects radio waves and sends them to a receiver, where the signal is detected ( Fig. 1.3). However, in 1932, Emerson and Arnold were only able to propose that a large number of chlorophyll molecules acted as a group to carry out photosynthesis, although it was not clear how this cooperation came about. The collection of chlorophyll molecules and associated enzymes came to be known as a photosynthetic unit,a term proposed by Gaffron and Wohl (1936). We will explore the details of the structure and function of antennas, reaction centers, and other components of the photosynthetic unit in later chapters.
Читать дальше