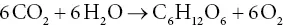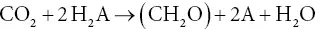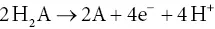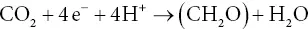(3.1) 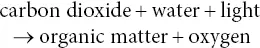
This was not yet a balanced equation, as the nature of the organic matter had not been established, and even the proper chemical formula for water had not yet been established! The concept of atoms combining to form molecules was just being formulated by John Dalton (1766–1844) near the end of this period, but unambiguous chemical formulas were not established for another 50 years.
It should be clear that the elucidation of the equation of photosynthesis was an amazing feat, which required contributions from many brilliant and hardworking scientists of differing backgrounds and points of view. It should also be clear from this example that science is very much an enterprise carried out by human beings, who are often blinded by preconceived ideas, ego, and jealousy.
3.6.1 The balanced equation for photosynthesis
It was another 60 years before the chemical equation of photosynthesis could be written in modern chemical symbols and properly balanced. A key aspect of this development was the determination of the redox state of the organic matter produced during photosynthesis. This involved careful measurements of the photosynthetic quotient, the ratio between the carbon dioxide assimilated and the oxygen produced. The first accurate measurements of the photosynthetic quotient were made in 1864 by a Frenchman, Jean Baptiste Boussingault (1802–1887). This determination consisted of measuring the volumes of CO 2taken up and O 2produced during photosynthesis. Boussingault found that the photosynthetic quotient was close to 1 for a number of plants. This established that the fixed carbon is at the redox level of carbohydrate (where the ratio of H to O is 2:1). Bolstering this view, Julius von Sachs, a German plant physiologist, found in the same year that the carbohydrate, starch, accumulates in leaves only when they are illuminated, and only in those parts of the leaf that are directly illuminated. This effect can be illustrated dramatically by actually printing photographs on leaves! The process is accomplished by taping a photographic negative over a leaf, illuminating it to form starch, extracting the pigments, and then developing the image by treating it with iodine, which forms a dark‐colored complex with starch. Remarkably high‐quality images can be obtained in this manner (Walker, 1992).
The information that the photosynthetic quotient is 1, and that the organic matter is a carbohydrate such as starch or sugar, allows us to write a minimally balanced equation for photosynthesis:
(3.2) 
where (CH 2O) is representative of a carbohydrate. One example of a carbohydrate is glucose, C 6H 12O 6, which makes the overall balanced photosynthetic equation:
(3.3) 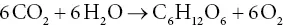
As we will see in Chapter 9, glucose is not the carbohydrate directly formed in photosynthesis, but it has almost the same energy content, so this is adequate for our present needs.
Julius Robert Mayer (1814–1878), a German physician and physicist who first enunciated the law of conservation of energy, proposed in 1845 that in photosynthesis light energy is converted to chemical energy, thus completing the formulation of the equation of photosynthesis.
3.7 Early mechanistic ideas of photosynthesis
When the overall reaction of photosynthesis had been established, attention turned to elucidating the details of the mechanism of the process. The early ideas in this regard were erroneous and much too simplistic. Richard Willstätter (1872–1942) and Arthur Stoll (1887–1971) proposed in 1918 that the product was actually formed directly as a molecular species, formaldehyde (CH 2O), in a direct, concerted process involving chlorophyll, CO 2, and H 2O. This view was revived later by Otto Warburg (1883–1970) in support of his unorthodox formulation of photosynthesis, which we will discuss a little later. We now know that these mechanistic ideas, which were certainly reasonable at the time, are not valid, because there are literally dozens of intermediate states that have been identified, and the reduction in CO 2can be separated from the production of oxygen, and vice versa. The key to understanding in more detail how the mechanism of photosynthesis really works came from the analysis of simple photosynthetic organisms by van Niel and by Hill's experiments showing that CO 2reduction and O 2evolution can be decoupled.
3.7.1 Van Niel and the redox nature of photosynthesis
The cornerstone of our current understanding of photosynthesis is that it is a light‐induced reduction–oxidation (redox) chemical process. This principle was first clearly set forth in the 1930s by the Dutch microbiologist Cornelis van Niel (1897–1985), working at Stanford University. Van Niel carried out a series of experiments on the metabolic characteristics of non‐oxygen‐evolving (anoxygenic) photosynthetic bacteria (van Niel, 1941). These organisms contain bacteriochlorophylls, pigments related to, but distinct from, the chlorophylls contained in cyanobacteria, algae, and plants. They assimilate CO 2into organic matter, but do not produce molecular oxygen. In order for these bacteria to assimilate CO 2, they must be supplied with a reducing compound. Many different compounds will suffice, most notably H 2S, which is first oxidized to elemental sulfur and then further oxidized. In place of H 2S, a variety of organic compounds can also be utilized, or even molecular hydrogen. Van Niel's seminal contribution was the recognition that these compounds could all be represented by the general formula H 2A, and that the overall equation of photosynthesis could be reformulated in a more general way as follows:
(3.4) 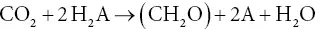
The oxygen‐evolving form of photosynthesis can then be seen as a special case of this more general formulation, in which H 2O is H 2A and O 2is 2A. When presented in this manner, the redox nature of photosynthesis is much more obvious. In fact, it is a simple further step to separate the oxidation and reduction into two chemical equations, one for the oxidation and the other for the reduction:
(3.5a) 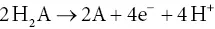
(3.5b) 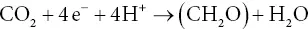
This separation into oxidation and reduction reactions leads to a number of important predictions. First, it suggests that the two processes might possibly be physically or temporally separated. Indeed, this was found to be the case by Hill in his classic experiments on the use of artificial electron acceptors, which will be discussed in the next paragraph. A second prediction is that the oxygen produced by oxygen‐evolving photosynthesis comes from H 2O and not from CO 2. This is indeed the case, although it took many years for this fact to be established unequivocally, because of the rapid exchange of oxygen between CO 2and H 2O via carbonic acid, H 2CO 3, which is constantly forming and breaking down. Van Niel correctly imagined that the oxidation and reduction reactions of Eqs. (3.5a)and (3.5b)were not the primary processes carried out by light, but were, rather, the result of a primary oxidant and reductant generated in the light, which went on to react with the substrates to form the products. However, some of van Niel's detailed ideas about the nature of the primary oxidant and reductant were incorrect, and he was overly rigid in insisting that all assimilated carbon had first to be oxidized all the way to CO 2before it could be subsequently reduced. Nevertheless, the truth of the basic principle of photosynthesis as a light‐induced redox process was unequivocally established by van Niel's work, along with that of other scientists of the time, notably Hans Gaffron (1902–1979).
Читать дальше