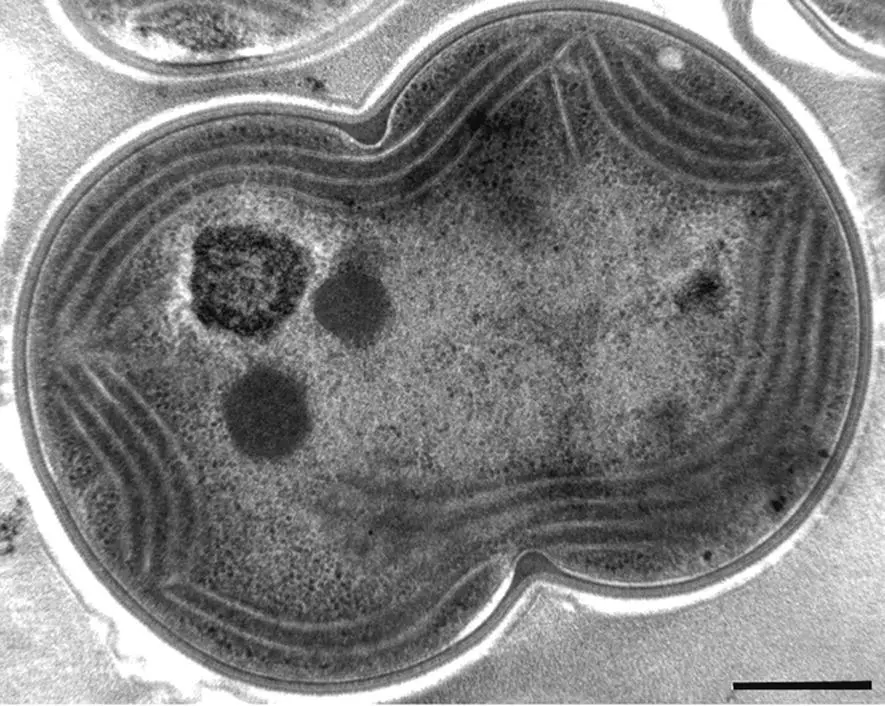
Figure 2.4 Thin section transmission electron micrograph of the cyanobacterium Synechocystis PCC 6803 prepared by high‐pressure cryofixation. Scale bar 0.4 μm.
Source: Courtesy of Robert Roberson.
Many species of cyanobacteria can fix nitrogen from N 2to NH 3, although, to do this, they face a special challenge. The enzyme system that fixes N 2, called nitrogenase, is very sensitive to O 2. The O 2produced by Photosystem II in cyanobacteria is therefore incompatible with N 2fixation. Cyanobacteria solve this problem in one of several different ways. In some filamentous forms, which grow as strings of cells, approximately every tenth cell will change its characteristics and become a special N 2‐fixing cell called a heterocyst(Wolk et al ., 1994). In these cells, Photosystem II is absent, an exceptionally thick cell wall inhibits diffusion of O 2into the cell, and O 2scavenging systems keep these cells anaerobic to protect the nitrogenase. The other major strategy employed is to carry out N 2fixation only when it is dark, when the cells are not producing O 2. An unusual group of nitrogen fixing cyanobacteria has lost all genes that code for Photosystem II and is an obligate symbiont with a eukaryotic alga (Thompson et al ., 2012).
A few groups of cyanobacteria can switch from using H 2O as an electron donor to using H 2S, with elemental sulfur as the product (Padan, 1979; Liu et al ., 2020). They are thus capable of true anoxygenic photosynthesis, although if H 2S is absent they produce O 2in much the same way as other cyanobacteria. The anoxygenic metabolism therefore represents an additional capability in these organisms, and they thus differ significantly from the other anoxygenic phototrophic prokaryotes, which cannot produce O 2under any environmental conditions.
Most cyanobacteria contain an extensive internal system of membranes called thylakoids. These membranes contain the photosynthetic apparatus (van de Meene et al ., 2006; Liberton et al ., 2011). All cyanobacteria contain chlorophyll a . Most species lack chlorophyll b and contain bilin pigments that are organized into large antenna complexes called phycobilisomes ( Chapter 5). A group of cyanobacteria, called prochlorophytes, contain chlorophyll b in addition to chlorophyll a (Matthijs et al ., 1994). This chlorophyll b ‐containing group might logically be assumed to be closely related to the organisms that became the chloroplasts of green algae and higher plants, which contain chlorophyll b . However, this relationship is not supported by analyses of some genetic markers (see below and Chapter 12). The chlorophyll b in these organisms is contained in antenna complexes that are structurally quite different from those of plant and algal chloroplasts. The prochlorophytes do not contain organized phycobilisomes, although some of them do contain genetic information for certain phycobiliproteins.
An important group of prochlorophytes is the genus Prochlorococcus (Partensky et al ., 1999). These cells are found in the deeper regions of the photic zone in the oceans. They were overlooked for many years because they are extremely small organisms, less than 1 μm in diameter, and passed through the holes in standard collection filters. Because of their small size, they are sometimes called picoplankton, along with other small marine cyanobacteria. They are also unusual in that the chlorophyll a present, divinyl chlorophyll a , is chemically slightly different from the chlorophyll a found in all other oxygenic phototrophs ( Chapter 4), a change that adapts them better to the photic environment where they are found. Other prochlorophytes include Prochloron , which was the first to be discovered. It grows as a symbiont with a marine animal known as an ascidian in the Great Barrier Reef off Australia and in other places in the South Pacific. Evidence strongly suggests that the prochlorophytes are polyphyletic in origin (Palenik and Haselkorn, 1992; Urbach et al ., 1992).
Two recently discovered groups of cyanobacteria are of particular interest. They contain the long‐wavelength‐absorbing pigments chlorophyll d and chlorophyll f , which absorb out to nearly 750 nm in the near infrared (Miyashita et al ., 1996; Chen et al ., 2010). These organisms live primarily in filtered light environments where other organisms above them absorb most of the visible light, so that only the near infrared radiation penetrates more deeply where these organisms live.
2.6 Photosynthetic eukaryotes
Eukaryotic photosynthetic organisms all contain the subcellular organelle called the chloroplast. An overview diagram of the photosynthetic complexes in the chloroplasts of a variety of photosynthetic eukaryotes is shown in Fig. 2.2(right panel). Chloroplasts are one of a larger group of organelles known as plastids, some of which carry out other functions, such as starch or pigment storage in flowers and fruits. As discussed above, a variety of evidence clearly shows that chloroplasts originated by a process called endosymbiosis, in which a cyanobacterial‐like cell was initially a symbiont with a protoeukaryotic cell and then eventually became a semiautonomous but essential part of the host cell (Margulis, 1993). The chloroplast contains DNA, which is organized and regulated in a manner typical of bacteria, not eukaryotes. This DNA encodes a number of chloroplast proteins that function in photosynthesis and chloroplast‐localized ribosomal protein synthesis machinery. After the initial endosymbiotic event, a significant degree of genetic transfer to the nucleus took place, so the chloroplast no longer contains enough information to be completely free of the nucleus. The majority of chloroplast proteins are therefore coded for by nuclear DNA, which is transcribed into RNA, the proteins synthesized on cytoplasmic ribosomes and then imported into the chloroplast. In addition, the plastid is the site of the early steps in lipid biosynthesis for the entire cell, so essential cellular components are also exported from the chloroplast. This division of labor requires a sophisticated control and regulation mechanism, which is discussed in more detail in Chapter 10. Mitochondria also originated by endosymbiosis, but in this case the symbiont was a proteobacterium instead of a cyanobacterium.
In addition to the primary endosymbiosis, which formed the first photosynthetic eukaryote, there is abundant evidence that there have been several secondary endosymbioses, in which a eukaryotic photosynthetic organism underwent a second endosymbiosis and in some cases even tertiary endosymbiosis (Keeling, 2013). Some of the classes of algae discussed below originated via this mechanism. The evolutionary relationships among all the types of photosynthetic organisms and the complex history of the various groups of eukaryotic photosynthetic organisms are discussed in more detail in Chapter 12.
An electron micrograph of a typical higher plant chloroplast is shown in Fig. 2.5. A schematic diagram of the chloroplast is shown in Fig. 2.6. The chloroplast has dimensions of a few microns, slightly larger than the size of a typical bacterium. It is surrounded by a chloroplast envelope, made up of a double membrane with two complete bilayers separated by an intermembrane space. The region inside the inner chloroplast envelope membrane is called the stroma. The stroma is like the cytoplasm of the chloroplast and contains numerous soluble enzymes, in particular the enzymes involved in carbon fixation. An extensive internal membrane system inside the chloroplast, called the thylakoid membrane, contains chlorophyll and the electron transport system that carries out the initial light energy capture and storage. In higher plants, these thylakoids are pressed together in multiple places to form collections of very densely packed membranes, called grana, which are in turn connected by other membranes that are not pressed together. These membranes are known as stroma lamellae. In cyanobacteria and many algae, the thylakoid membranes are not found together in densely stacked grana, but are instead associated in stacks of two or a few membranes. As we will learn in more detail in Chapter 7, the components of the photosynthetic apparatus in algae and plants are not uniformly distributed in the thylakoid membranes. Photosystem II is localized primarily in the grana membranes, whereas Photosystem I is found mostly in the stroma lamellae. The thylakoid membranes appear in many pictures to be arranged like a stack of coins. However, in reality, they are highly interconnected, and actually form one or a few interconnected membranes, as shown in Fig. 2.7(Daum and Kühlbrandt, 2011). Like all biological membranes, the thylakoid is intrinsically asymmetric, with the components arranged with a particular vectorial orientation in the membrane. This results in an overall sidedness to the thylakoid membrane system. The side of the thylakoid that is toward the stroma is called the stromal side, whereas the enclosed space that is in contact with the opposite side of the thylakoid is called the lumen. This distinction between the two sides of the thylakoid membrane is a crucial point, as many of the functions of the chloroplast components rely on the presence of a membrane system that is osmotically intact and impermeable to ions.
Читать дальше













