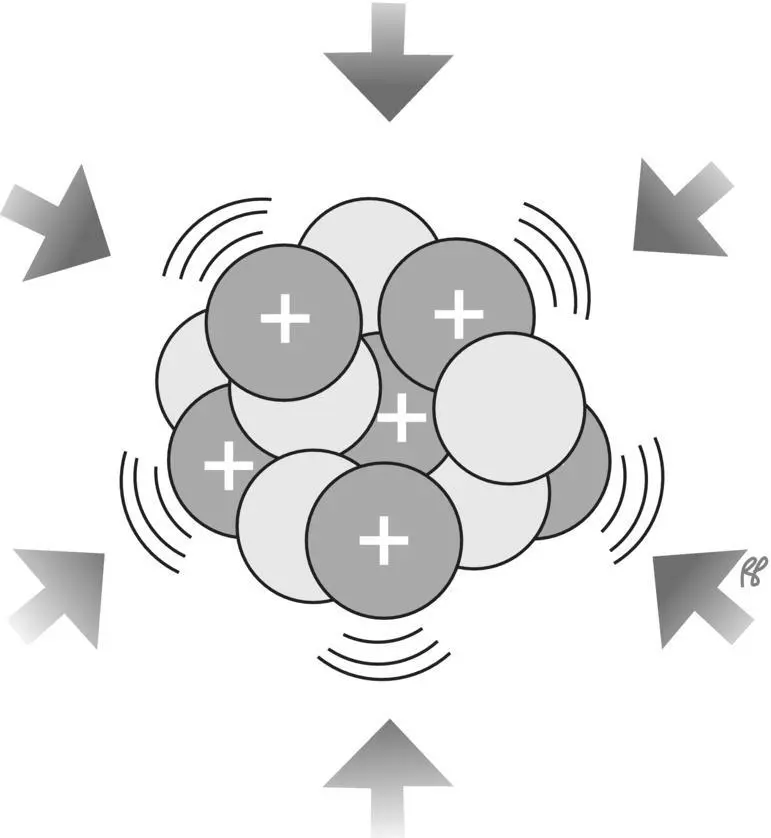
Figure 1.9 Nuclear binding force is strong enough to overcome the electrical repulsion between the positively charged protons.
Isotopes, isotones, and isobars:
Each atom of any sample of an element has the same number of protons (the same Z: atomic number) in its nucleus. Lead found anywhere in the world will always be composed of atoms with 82 protons. The same does not apply, however, to the number of neutrons in the nucleus.
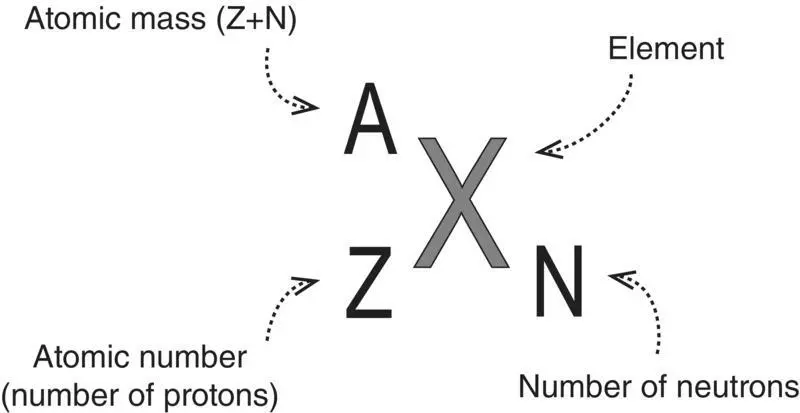
Figure 1.10 Standard atomic notation.
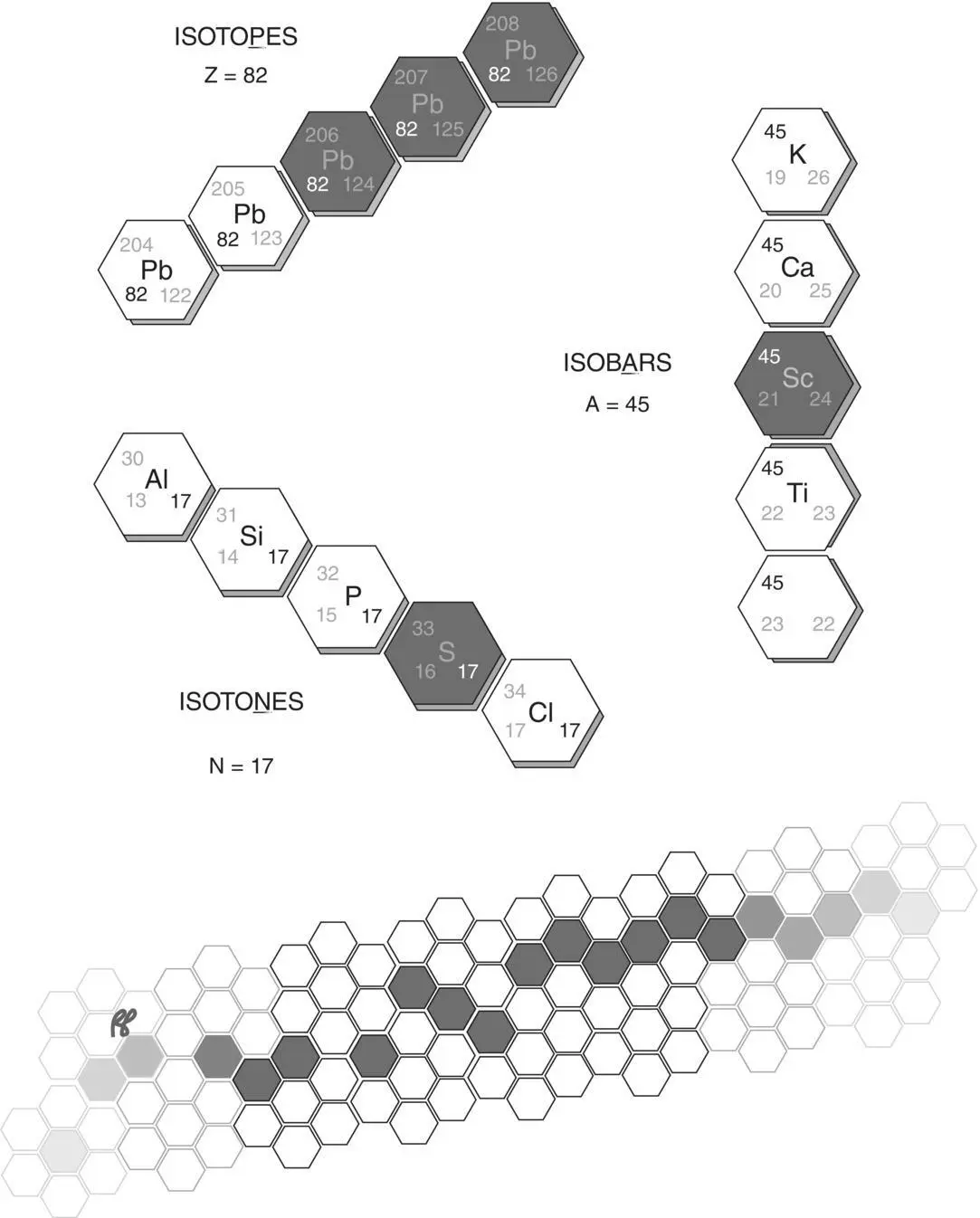
Figure 1.11 Nuclides of the same atomic number but different atomic mass are called isotopes, those of an equal number of neutrons are called isotones, and those of the same atomic mass but different atomic number are called isobars. Stable nuclear configurations are shaded gray, radioactive configurations are white.
(Adapted from Brucer, M. Trilinear Chart of the Nuclides, Mallinkrodt Inc, 1979.)
An isotopeof an element is a particular variation of the nuclear composition of the atoms of that element. The number of protons ( Z: atomic number) is unchanged, but the number of neutrons ( N) varies. Since the number of neutrons changes, the total number of neutrons and protons ( A: the atomic mass) changes. The chemical symbol for each element can be expanded to include these three numbers ( Figure 1.10).
Two related entities are isotonesand isobars. Isotones are atoms of different elements that contain identical numbers of neutrons but varying numbers of protons. Isobars are atoms of different elements with identical numbers of nucleons. Examples of these are illustrated in Figure 1.11. Nuclideis a general term for the composition of a nucleus and includes isotopes, isotones, isobars, and other nuclear configurations.
Not all elements have stable isotopes; they do exist for most of the light and mid‐weight elements, those with atomic numbers (number of protons) up to and including bismuth ( Z = 83). However, there are no stable isotopes of technetium ( Z = 43), promethium ( Z = 61), or for all elements with atomic numbers higher than 83. Prominent examples are radium ( Z = 88) and uranium ( Z = 92), which are found naturally as a mix of isotopic forms that are all radioactive.
For those nuclei with a stable state there is an optimal ratio of neutrons to protons. For the lighter elements this ratio is approximately 1:1; for increasing atomic weights, stability is more likely when the number of neutrons exceeds the number of protons. A plot depicting the number of neutrons as a function of the number of protons is called the line of stability( Figure 1.12).
Strictly speaking, stability is a relative term. We call a nuclide stable when its half‐life is so long as to be practically immeasurable—say greater than 100 years. An isotope of potassium, 40K for example, which makes up about 1% of the potassium found in nature is considered stable but actually has a half‐life of 10 9years.
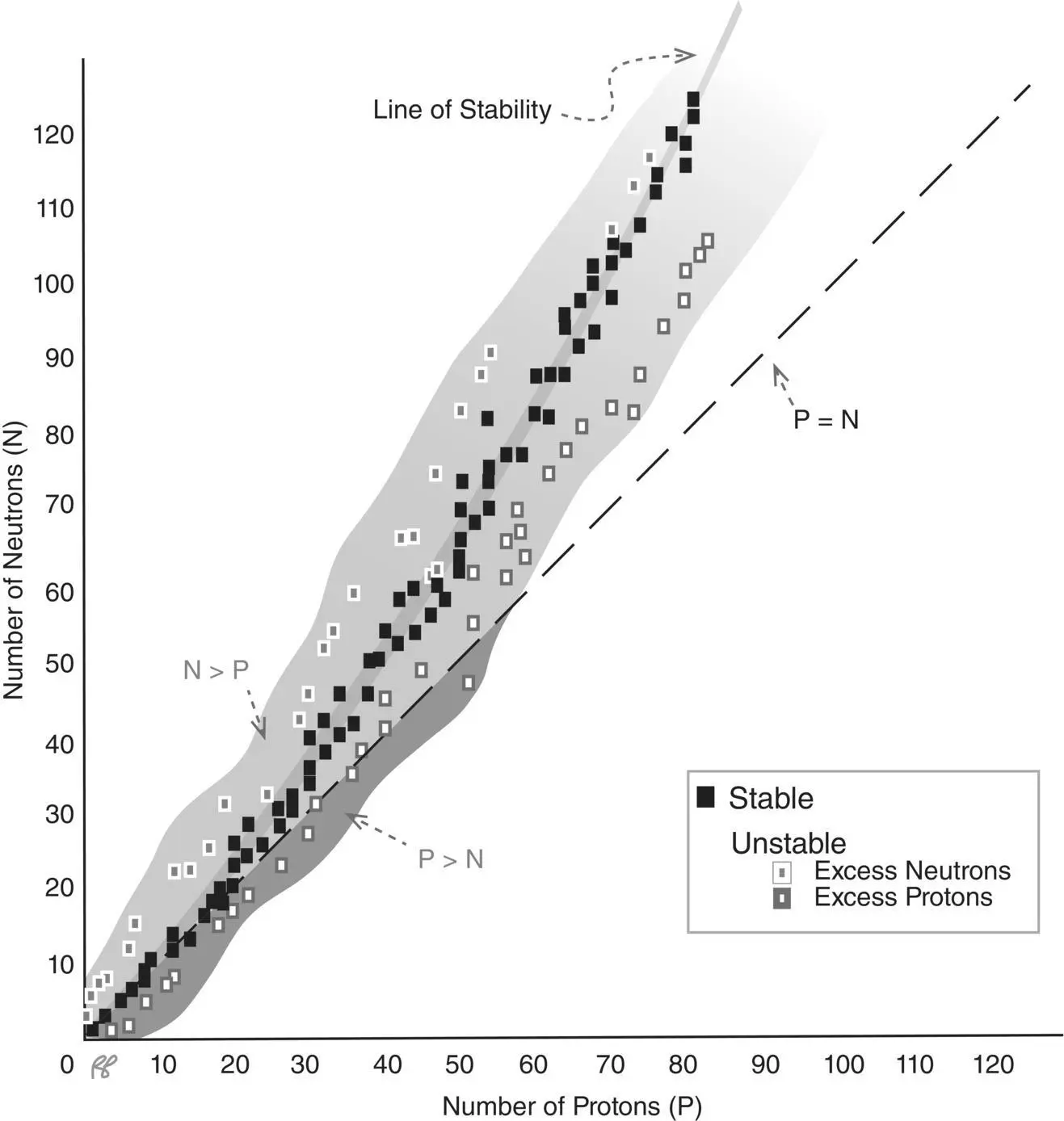
Figure 1.12 Combinations of neutrons and protons that can coexist in a stable nuclear configuration all lie within the gray shaded regions.
Radioactivity
The unstable nucleus and radioactive decay
A nucleus which is not in its stable state will adjust itself until it is more stable either by ejecting portions of its nucleus or by emitting energy in the form of photons (gamma rays). This process is referred to as radioactive decay. The type of decay depends on which of the following rules for nuclear stability is violated.
Excessive nuclear mass
Alpha decay:
Very large unstable atoms, atoms with high atomic mass, may split into nuclear fragments. The smallest stable nuclear fragment that is emitted is the particle consisting of two neutrons and two protons, equivalent to the nucleus of a helium atom. Because it was one of the first types of radiation discovered, the emission of a helium nucleus is called alpha radiation, and the emitted helium nucleus an alpha particle( Figure 1.13).
Under some circumstances, the nucleus of the unstable atom may break into larger fragments, a process usually referred to as nuclear fission. During fission two or three neutrons are emitted ( Figure 1.14).
Unstable Neutron–Proton Ratio
Too many neutrons—beta decay:
Nuclei with excess neutrons can achieve stability by a process that amounts to the conversion of a neutron into a proton and an electron. The proton remains in the nucleus, but the electron is emitted. This is called beta radiation, and the electron itself a beta particle( Figure 1.15). The process and the emitted electron were given these names to contrast with the alpha particle before the physical nature of either was discovered. The beta particle generated in this decay will become a free electron until it finds a vacancy in an electron shell either in the atom of its origin or in another atom.
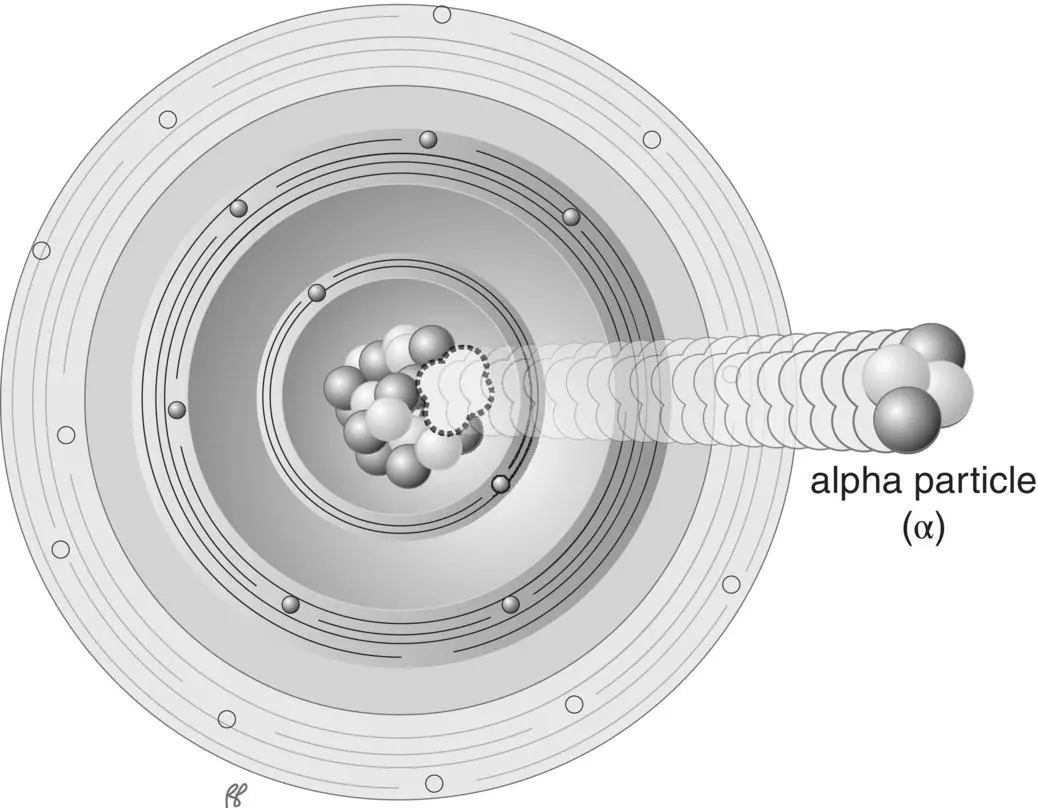
Figure 1.13 Alpha decay.
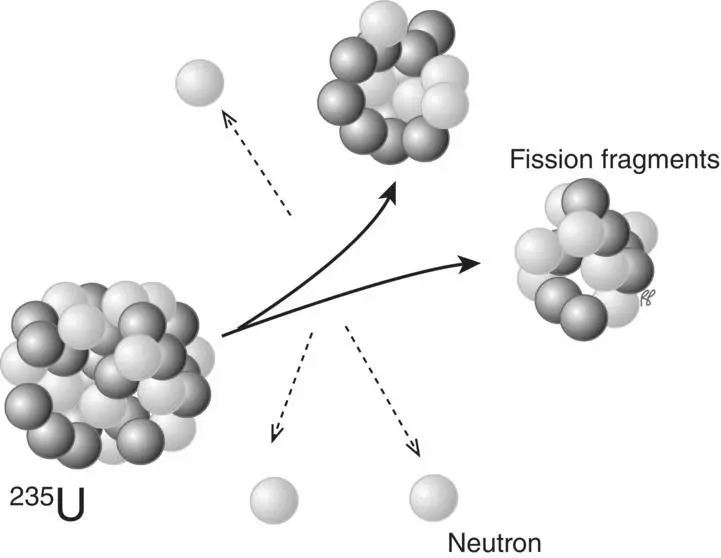
Figure 1.14 Fission of a 235U nucleus.
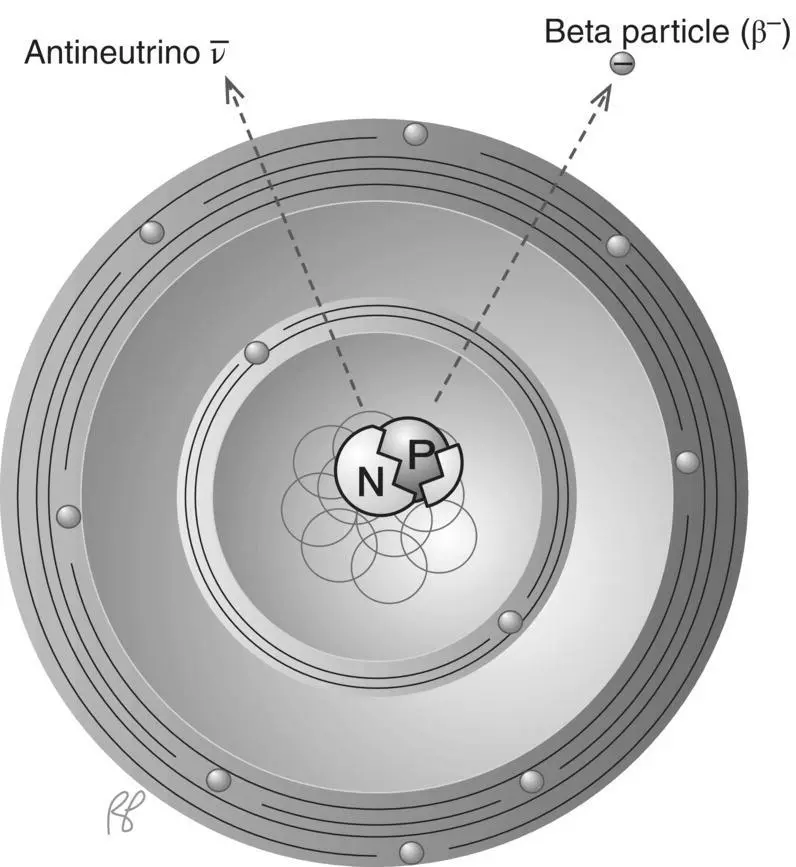
Figure 1.15 β –(negatron) decay.
Careful study of beta decay suggested to physicists that the conversion of neutron to proton involved more than the emission of a beta particle (electron). Beta emission satisfied the rule for conservation of charge in that the neutral neutron yielded one positive proton and one negative electron; however, it did not appear to satisfy the equally important rule for conservation of energy. Measurements showed that most of the emitted electrons simply did not have all the energy expected. To explain this apparent discrepancy, the emission of a second particle was postulated and that particle was later identified experimentally. Called an antineutrino(neutrino for small and neutral), it carries the “missing” energy of the reaction.
Too many protons—positron decay and electron capture:
Читать дальше



















