Rachel A. Powsner - Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology
Здесь есть возможность читать онлайн «Rachel A. Powsner - Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
The molecule is held together by the chemical bonds among its atoms. These bonds are formed by the force of electrical attraction between oppositely charged parts of the molecule. This force is often referred to as the Coulomb force after Charles A. de Coulomb, the physicist who characterized it. This is the force involved in chemical reactions such as the combining of hydrogen and oxygen to form water. The electrons of the atom are held by the electrical force between them and the positive nucleus. The nucleus of the atom is held together by another type of force—nuclear force—which is involved in the release of atomic energy. Nuclear forces are magnitudes greater than electrical forces.
Elements
There are more than 100 species of atoms. These species are referred to as elements. Most of the known elements—for example, mercury, helium, gold, hydrogen, and oxygen—occur naturally on earth; others are not usually found in nature but are made by humans—for example, europium and americium. A reasonable explanation for the absence of some elements from nature is that if and when they were formed they proved too unstable to survive in detectable amounts into the present.

Figure 1.1 Electrostatic charge.
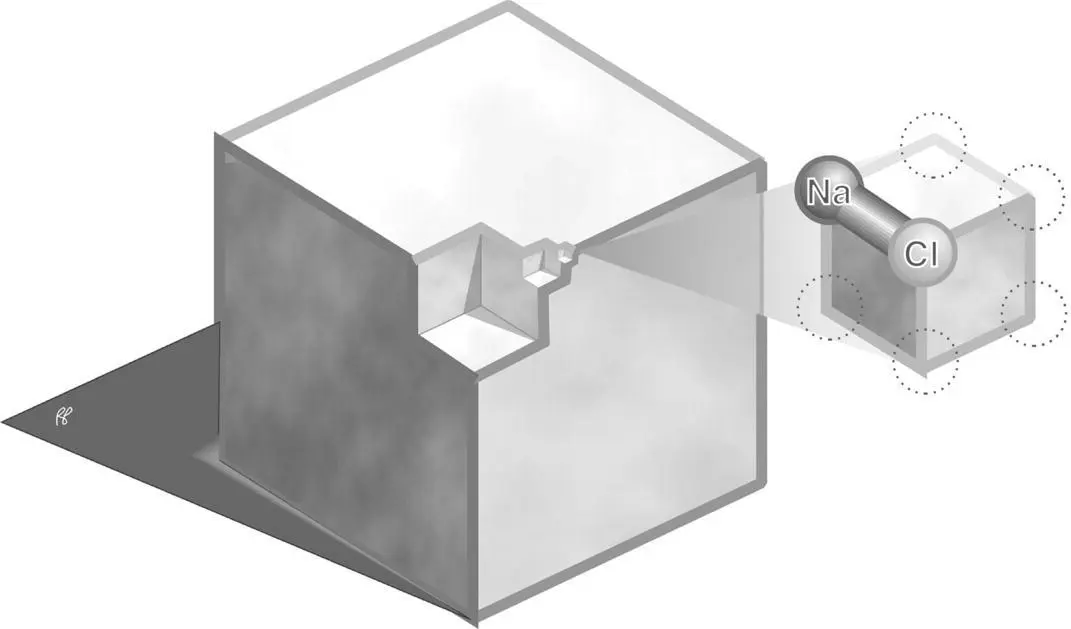
Figure 1.2 The NaCl molecule is the smallest unit of salt that retains the characteristics of salt.
All the elements have been assigned symbols or abbreviated chemical names: gold, Au, mercury, Hg; helium, He. Some symbols are obvious abbreviations of the English name; others are derived from the original Latin name of the element, for example, Au is from aurum, the Latin word for gold.
All of the known elements, both natural and those made by humans, are organized in the periodic table. In Figure 1.3, the elements that have a stable state are shown in white boxes; those that occur only in a radioactive form are shown in gray boxes. The number appearing above each element’s abbreviation is referred to as the atomic number, which will be discussed later in this chapter.

Figure 1.3 Periodic table.
The elements in the periodic table are arranged in columns (called groups) and rows (called periods). In general, elements within groups demonstrate similar properties. This is because elements in a group often have similar numbers of electrons in their outer shell; outer shell electron configurations are more important in determining how an atom interacts with other elemental atoms. The lanthanides and actinides are special groups of elements, conventionally shown in rows, separated and placed below the table. These two groups have the same number of outer‐shell electrons and share many common properties.
Atomic structure
Atoms initially were thought of as no more than small pieces of matter. Our understanding that they have an inner structure has its roots in the observations of earlier physicists that the atoms of which matter is composed contain electronsof negative charge. In as much as the atom as a whole is electrically neutral, it seemed obvious that it must also contain something with a positive charge to balance the negative charge of the electrons. Thus, early attempts to picture the atom, modeled on our solar system, showed the negatively charged electrons orbiting a central group of particles, the positively charged nucleus( Figure 1.4).
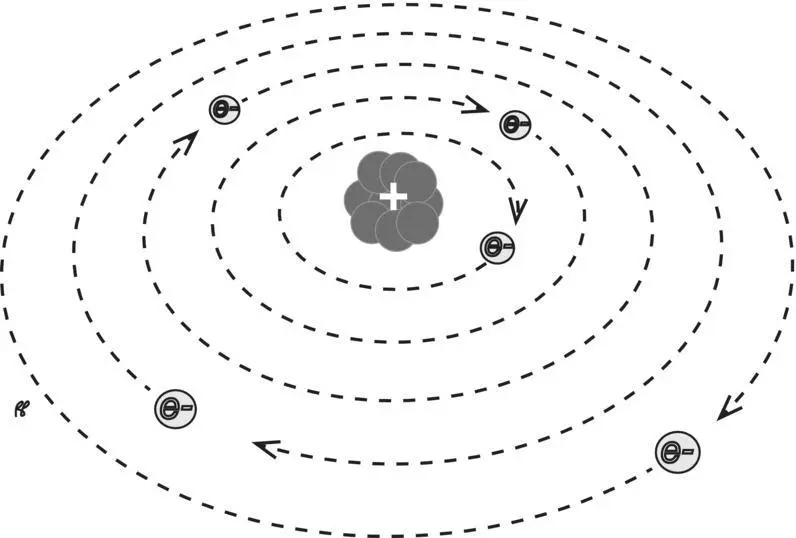
Figure 1.4 Flat atom. The standard two‐dimensional drawing of atomic structure.
Electrons
In our simple solar‐system model of the atom, the electrons are viewed as orbiting the nucleus at high speeds. They have a negative charge and the nucleus has a positive charge. The electrical charges of the atom are “balanced,” that is, the total negative charge of the electrons equals the positive charge of the nucleus. As we shall see in a moment, this is simply another way to point out that the number of orbital electrons equals the number of nuclear protons.
Electron shells and binding energy:
By adding a third dimension to our model of the atom, we can depict the electron orbits as the surfaces of spheres (called shells) to suggest that, unlike the planets orbiting the sun, electrons are not confined to a circular orbit lying in a single plane but may be more widely distributed ( Figure 1.5). Although it is convenient for us to talk about distances and diameters of the shells, distance on the atomic scale does not have quite the same meaning it does with everyday objects. The most significant characteristic of a shell is its energy level. The “closer” an electron is to the nucleus, the more tightly it is bound to the nucleus. In saying this, we mean that more work (energy) is required to remove an inner‐shell electron than an outer one. The energy that must be put into the atom to separate an electron is called the electron binding energy. It is usually expressed in electron volts (eV). The electron binding energy varies from a few thousand electron volts (keV) for inner‐shell electrons to just a few eV for the less tightly bound outer‐shell electrons.
Electron volt
The electron volt is a special unit defined as the energy required to move one electron against a potential difference of one volt. Conversely it is also the amount of kinetic (motion) energy an electron acquires if it “falls” through a potential difference of one volt. It is a very small unit on the everyday scale, at only 1.6 × 10 –19joules (J), but a very convenient unit on the atomic scale. One joule is the Système International (SI) unit of work or energy. For comparison, 1 J equals 0.24 small calories (as opposed to the kcal used to measure food intake).
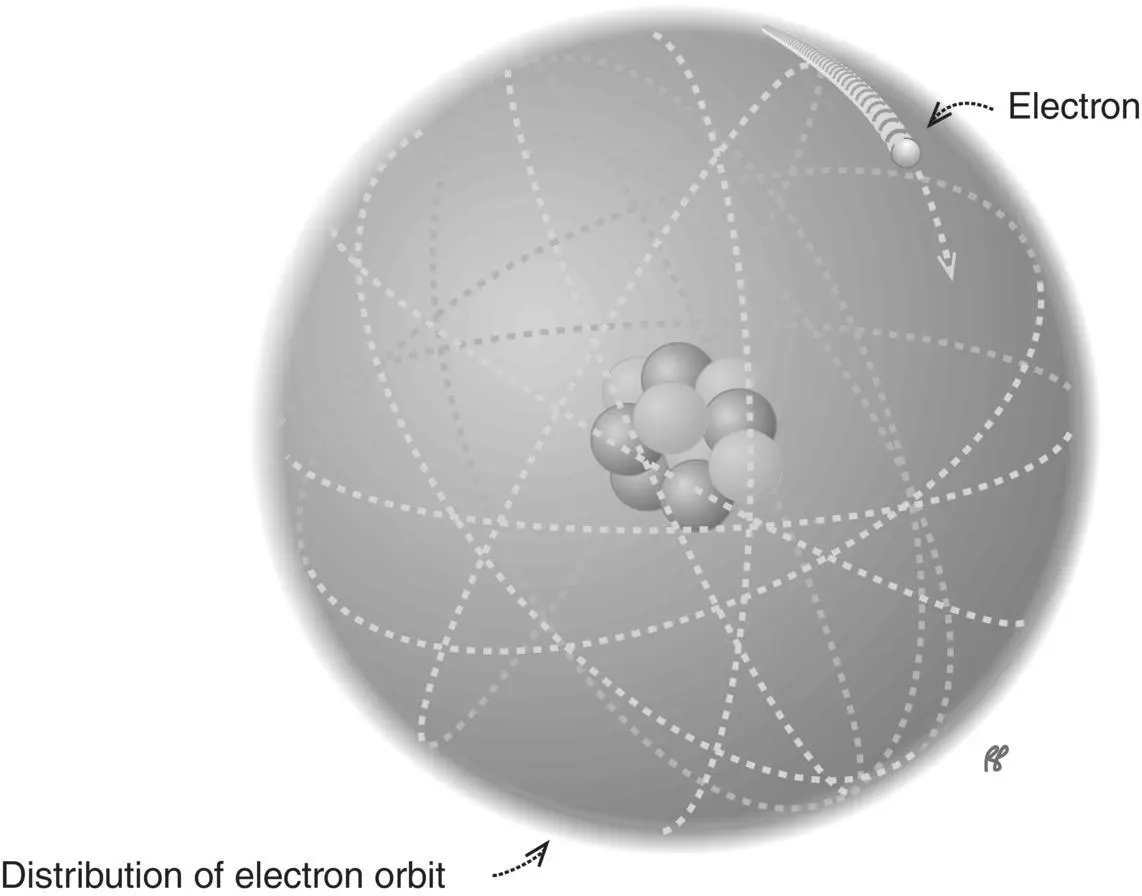
Figure 1.5 An electron shell is a representation of the energy level associated with an atomic electron.
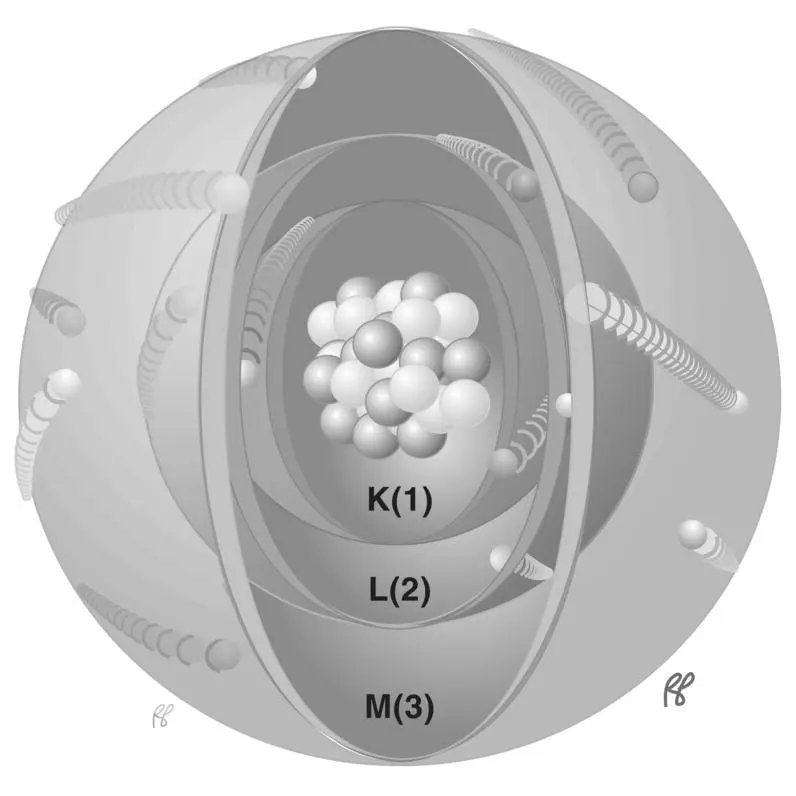
Figure 1.6 K, L, and M electron shells.
Quantum numbers:
The atomic electrons in their shells are usually described by their quantum numbers, of which there are four types. The first is the principal quantum number( n), which identifies the shell. The first three shells (K, L, and M) are depicted in Figure 1.6. The electron binding energy is greatest for the innermost shell (K) and is progressively less for the outer shells. Larger atoms have more shells.
The second (azimuthal), third (magnetic), and fourth (spin) quantum numbers refer to other physical properties of the electron. Each electron within an atom has a unique combination of the four quantum numbers.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology»
Представляем Вашему вниманию похожие книги на «Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












