Rachel A. Powsner - Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology
Здесь есть возможность читать онлайн «Rachel A. Powsner - Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
The maximum number of electrons associated with each energy shell is 2 n 2, where n is the shell number. The first shell (the K shell) can contain a maximum of two electrons, the second shell (the L shell) can contain a maximum of eight electrons, the third shell (the M shell) can contain a maximum of 18 electrons, and so on.
Representation of electron distribution:
Most of the diagrams (for example Figure 1.6) in this chapter reflect what is referred to as the Bohr modelof the atom and as such all electrons within each shell are depicted as moving along the surface of a sphere, each shell represented as one such sphere with a distinct radial distance from a centrally located nucleus. The radius of these spheres increases with principal quantum number. This model of the atom is frequently used for teaching purposes because the radial distance of an electron from the nucleus is used to depict with how tightly bound it is to the atom—the closest electrons being most tightly bound.
A more accurate quantum mechanical description of electron distribution uses a sequence of orbitals. Orbitals are mathematical functions that describe the probability of finding an electron in a region of space near the nucleus. For each principal quantum number (each shell) there is a spherical orbital denoted by the principal quantum number followed by the letter “s”. This orbital contains two electrons ( Figure 1.7a). This is the only orbital for the K shell which contains at maximum two electrons and this orbital is called the 1s orbital.The neutral atom with a full K shell is the helium atom.
The next shell, the L shell ( n = 2), also has a spherical orbital, denoted 2s (also depicted as Figure 1.7a) which contains two electrons, as well as three sub‐orbitals, denoted 2p x, 2p y, 2p z. Each sub‐orbital has a shape like a dumb‐bell or three‐dimensional figure eight (see Figure 1.7b). The three sub‐orbitals are oriented along three orthogonal axes as shown in Figure 1.7c. Each sub‐orbital is filled by two electrons and the neutral atom with completely filled orbitals for n = 1 and n = 2 is Neon.
For the higher order orbitals, n > 2, the sub‐orbitals associated with higher azimuthal quantum number become even more complicated in structure and will not be discussed here.
Quantum numbers
The term quantummeans, literally, amount. It acquired its special significance in physics when Bohr and others theorized that physical quantities such as energy and light could not have a range of values as on a continuum, but rather could have only discrete, step‐like values. The individual steps are so small that their existence escaped the notice of physicists until Bohr postulated them to explain his theory of the atom. We now refer to Bohr’s theory as quantum theoryand the resulting explanations of motion in the atomic scale as quantum mechanicsto distinguish it from the classical mechanics described by Isaac Newton, which is still needed for everyday engineering.
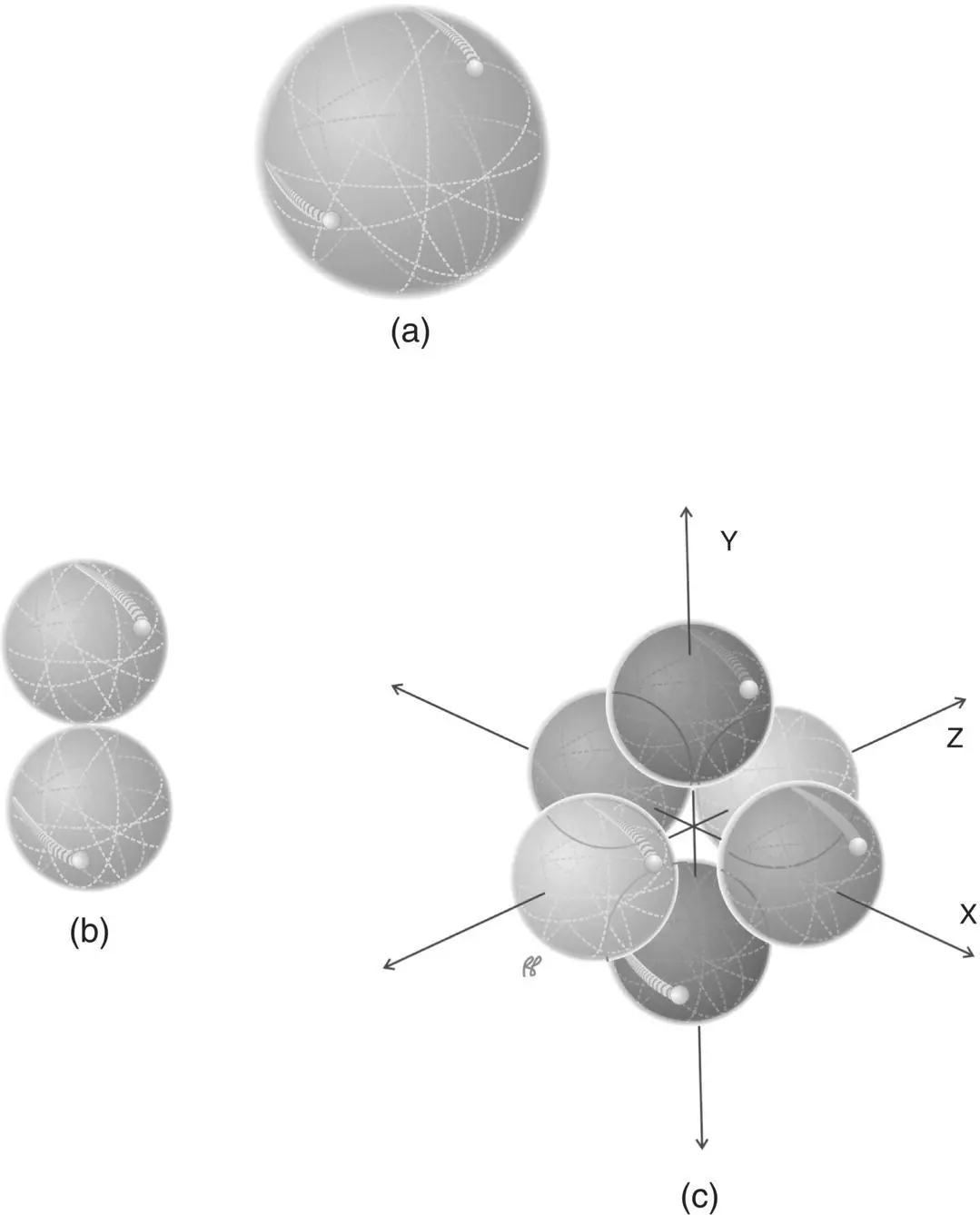
Figure 1.7 Electron orbitals and sub‐orbitals. (a) s orbital, (b) p suborbital, (c) p suborbitals, p x, p y, p z.
Stable electron configuration:
Just as it takes energy to remove an electron from its atom, it takes energy to move an electron from an inner shell to an outer shell, which can also be thought of as the energy required to pull a negative electron away from the positively charged nucleus. Any vacancy in an inner shell creates an unstable condition often referred to as an excited state.
The electrical charges of the atom are balanced, that is, the total negative charge of the electrons equals the total positive charge of the nucleus. This is simply another way of pointing out that the number of orbital electrons equals the number of nuclear protons. Furthermore, the electrons must fill the shells with the highest binding energy first. At least in the elements of low atomic number, electrons within the inner shells have the highest binding energy.
If the arrangement of the electrons in the shells is not in the stable state, they will undergo rearrangement in order to become stable, a process often referred to as de‐excitation. Because the stable configuration of the shells always has less energy than any unstable configuration, the de‐excitation releases energy as X‐rays and electrons (this will be discussed in more detail later in this chapter in the section on internal conversion).
Nucleus
Like the atom itself, the atomic nucleus also has an inner structure ( Figure 1.8). Experiments showed that the nucleus consists of two types of particles: protons, which carry a positive charge, and neutrons, which carry no charge. The general term for protons and neutrons is nucleons. The nucleons have a much greater mass than electrons. Table 1.1reviews the properties of the various subatomic particles.
A simple but useful model of the nucleus is a tightly bound cluster of protons and neutrons. Protons naturally repel each other since they are positively charged; however, there is a powerful binding force called the nuclear forcethat holds the nucleons together very tightly ( Figure 1.9). The work (energy) required to overcome the nuclear force, the work to remove a nucleon from the nucleus, is called the nuclear binding energy. Typical binding energies are in the range of 2 million to 9 million electron volts (MeV) (approximately one thousand to one million times the electron binding force). The magnitude of the binding energy is related to another fact of nature: the measured mass of a nucleus is always less than the mass expected from the sum of the masses of its neutrons and protons. The “missing” mass is called the mass defect, the energy equivalent of which is equal to the nuclear binding energy. This interchangeability of mass and energy was immortalized in Einstein’s equation E = mc 2.
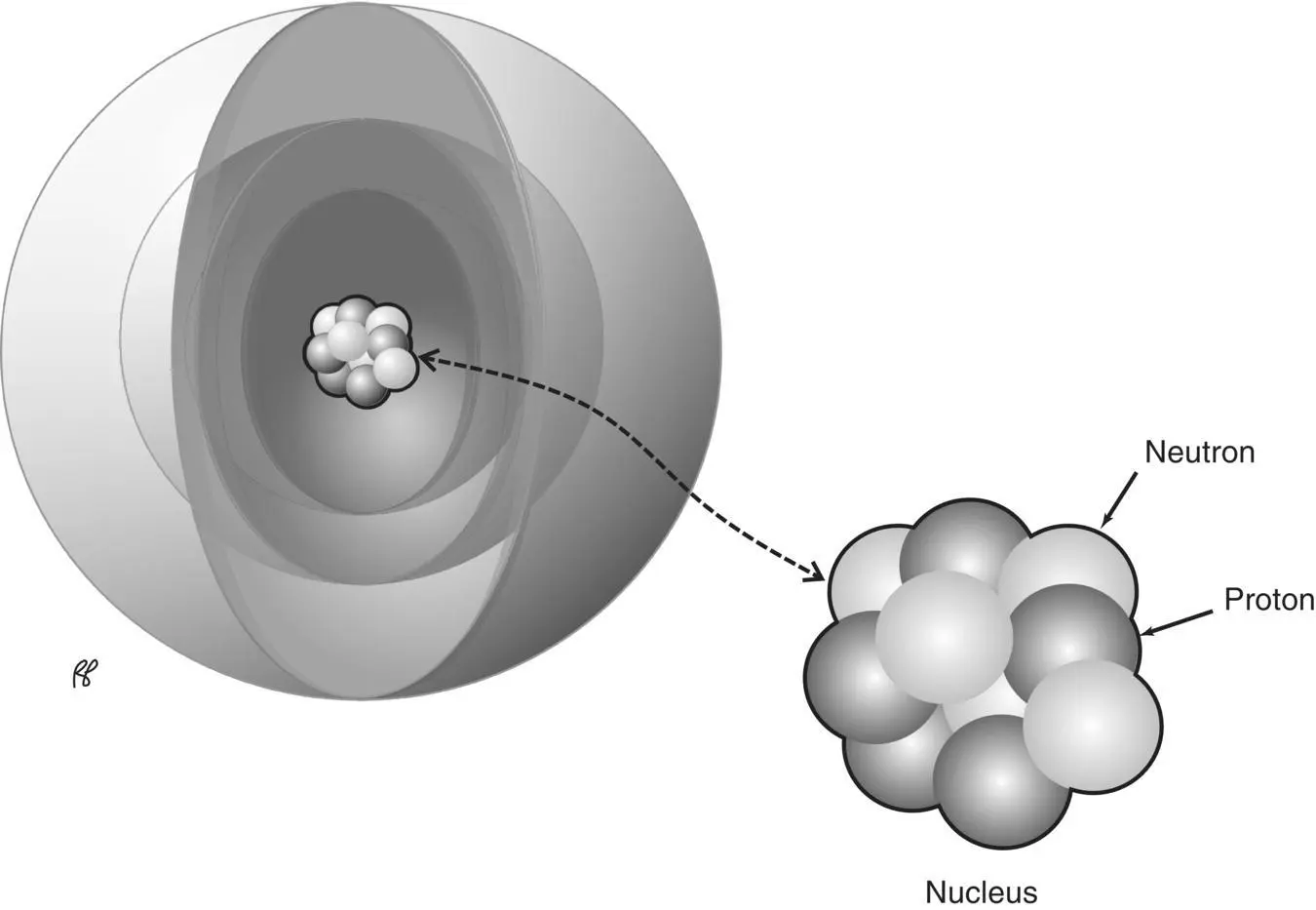
Figure 1.8 The nucleus of an atom is composed of protons and neutrons.
Table 1.1 Properties of the subatomic particles
| Name(s) | Symbol | Mass a | Charge |
|---|---|---|---|
| Neutron | N | 1839 | None |
| Proton | P | 1836 | Positive (+) |
| Electron | e – | 1 | Negative (–) |
| Beta particle (beta minus particle, electron) b | Β– | 1 | Negative (–) |
| Positron (beta plus particle, positive electron) | β+ | 1 | Positive (+) |
| Gamma ray (photon) | γ | None | None |
| X‐ray | X‐ray | None | None |
| Neutrino | ν | Near zero | None |
| Antineutrino | 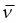 |
Near zero | None |
aRelative to an electron.
bThere is no physical difference between a beta particle and an electron; the term beta particle is applied to an electron that is emitted from a radioactive nucleus. The symbol β without a minus or plus sign attached always refers to a beta minus particle or electron.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology»
Представляем Вашему вниманию похожие книги на «Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












